Volume 31, Number 8—August 2025
Dispatch
Genomic Surveillance Detection of SARS-CoV-1–Like Viruses in Rhinolophidae Bats, Bandarban Region, Bangladesh
Cite This Article
Citation for Media
Abstract
We sequenced sarbecovirus from Rhinolophus spp. bats in Bandarban District, Bangladesh, in a genomic surveillance campaign during 2022–2023. Sequences shared identity with SARS-CoV-1 Tor2, which caused an outbreak of human illnesses in 2003. Describing the genetic diversity and zoonotic potential of reservoir pathogens can aid in identifying sources of future spillovers.
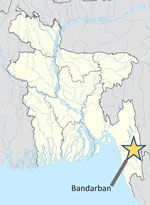
Zoonotic disease risk is influenced by various factors, including reservoir host density and distribution, pathogen prevalence, pathogen release, host/human proximity, and ability to infect and spread through spillover between species (1). Bats are well-known coronavirus reservoirs in Southeast Asia and are candidates for genomic surveillance for potential zoonotic transmission. In other ecosystems, climate and abiotic stressors can cause proximal shifts in bat roost sites, bringing bats into contact with domestic animals where virus spillover, including Hendra virus spillover, can occur (2,3; J. Lagergren et al., unpub. data, https://www.biorxiv.org/content/10.1101/2023.12.01.569640v1). Therefore, surveillance among bats colocated with bridging hosts are critical for defining spillover risk in any given region. We report preliminary results from genomic surveillance efforts focused on Rhinolophus spp. bat colonies at roost sites within the region of Bandarban, Bangladesh (Figure 1). We obtained study approval from the Bangladesh Forest Department and the Research Review Committee and Animal Experimentation Ethics Committee of the icddr,b (research protocol no. PR-20058, bat capture permit no. 22.01.0000.101.23.136.21.1088).
As part of our overall field campaign, we collected fecal samples from 240 Rhinolophus pusillus bats, 20 each month, during May 2022–April 2023. We captured only Rhinolophus spp. bats and released other bat species immediately after capture. A trained veterinarian collected all the samples after anesthetizing the bats, and all the bats were released at the site of capture within 2–5 hours of capture.
We assessed sex, weight, and health of individual bats before collecting a fecal sample and, in some cases, a blood sample from the radial vein/wing vein for immunological cell counts. For our sequencing studies, we selected fecal samples from a mix of female, male, juvenile, and adult bats with different body condition scores, choosing bats with higher leukocyte counts if those data were available. We inactivated the fecal samples by using TRIzol (Thermo Fisher Scientific, https://www.thermofisher.com) and later selected a subset of 12 fecal samples from summer 2022 (Table), representing 2 roosting locations (sites 1 and 2) in Bandarban, for initial screening on an iSeq (Illumina, https://www.illumina.com). We then performed deep sequencing on those samples by using a NextSeq 500 (Illumina). We enriched libraries by using the Comprehensive Viral Research Panel (Twist Bioscience, https://www.twistbioscience.com) supplemented with a custom-designed Chiropteran virus enrichment panel containing 134,000 probes. All sequencing was performed in a US Biosafety Level 4 facility. We deposited data into the National Center for Biotechnology Information Sequence Read Archive (submission no. SUB15226189 and BioProject no. PRJNA1249517).
We identified coronavirus sequence reads in 3 samples: B3, B4, and B6. The strongest signal was in B4, which comprised 1.2% of total reads and 5.5% of classified reads in the entire B4 sample (Table; Appendix Figures 1–3). We characterized virome components by using KRAKEN2 (4) and RefSeq viral database version April 2023 (Illumina). The B4-derived coronavirus sequences initially had 76.7% BLASTn (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) identity to SARS-CoV-1 Tor2 (Appendix Figure 4), which was isolated in 2003 from a patient who traveled from Hong Kong, China, to Toronto, Ontario, Canada, and who was hospitalized with febrile respiratory illness (5).
We further compared B4 to Tor2 and relatives by creating similarity plots in SimPlot++ version 1.3 (https://github.com/Stephane-S/Simplot_PlusPlus), and creating maximum-likelihood phylogenetic trees aligning the whole genome, RNA-dependent RNA polymerase, and spike sequences by using MAFFT version 7.508 (https://mafft.cbrc.jp/alignment/software) and IQ-TREE version 2.3.6 (http://iqtree.cibiv.univie.ac.at) (Appendix Figures 4, 5) (6–11). We found 2 notable dropouts in the alignment to SARS-CoV-1 Tor2: a 1-kb gap at nonstructural protein (NSP) 2 and a 2.1-kb gap over most of the spike receptor-binding domain (RBD) (Appendix Figure 4). To obtain complete genome coverage, we designed 2 primer pairs for each gap and used those primers to generate and sequence amplicons (Appendix Table 1). Initial amplicon analysis using BLASTn provided GenBank accession no. KY417143.1, bat SARS-like coronavirus isolate RS4081, which shared 85%–94% identity over a 99% query length. The main difference was the spike region (85% identity), which had no hits for 214 nt. A BLASTx (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastx&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome) query of the spike-specific amplicon indicated 76.2% identity (182 mismatches and 8 gaps) over a 97% query length to GenBank accession no. QVN46559.1, a spike glycoprotein from bat SARS-like coronavirus Khosta-1. A top BLASTx hit for the NSP2-specific amplicon was protein sequence NP_828861.2, an NSP2 of SARS-CoV-1 Tor2, which had 72.5% identity over a 99% query length. Further phylogenetic analysis of whole genomes, spike, and RNA-dependent RNA polymerase supported a novel virus with close spike homology to Sarbecovirus spp. (Appendix Figure 5).
We used AlphaFold modeling (European Molecular Biology Laboratory, European Bioinformatics Institute, https://alphafold.ebi.ac.uk) to compare the RBD of B4 with SARS-CoV-1 Tor2 RBD (Appendix Figure 6). Folding indicated similar shape and functionality and exhibited nonsynonymous substitutions and insertions. Two insertions were asparagine dimers, located on an edge likely to interact with mammalian angiotensin converting enzyme 2 (ACE2), and 1 insertion was a threonine located on another edge, making the B4 RBD sample structurally close to a sample from a known zoonotic human outbreak.
The binding of virus RBD to the primary receptor ACE2 is necessary for spillover infection to occur. We used a synthetic Förster resonance energy transfer–based assay (12) to test the binding affinity of known RBDs and the B4-derived RBD from our genomic surveillance data (Appendix Figure 7). We used ACE2 receptors from a variety of sympatric mammals (Appendix Table 2), including species that might reside near our bat sampling sites, such as Rattus spp. rats, Leopard cats (Prionailurus bengalensis), and humans (13). We chose ACE2 of the Etruscan shrew (Suncus etruscus) , which had sequence available for protein derivation, as a representative Suncus species for testing. That species has not specifically been observed yet in Bandarban, but its close relative, the S. murinus shrew, is widespread there and throughout Bangladesh. Dissociation constants for the novel B4 and 10 other bat coronavirus RBDs showed moderate binding of B4 to several native sympatric animals (Figure 2, panel A).
We were also interested in the potential of the Tor2 homologous B4-derived virus to infect humans. Therefore, we evaluated binding affinity relative to the 2019 wild-type SARS-CoV-2 RBD (Figure 2, panel B). The B4-derived RBD demonstrated approximately one third the binding efficiency of the wild-type strain, which was similar to results for other tested bat coronaviruses not yet detected in humans.
In 2019, a Tor2 analog was described in a bat reservoir in Korea (14), indicating the viral homologue may be regionally widespread from Bangladesh to southern China and the Korean Peninsula. Abiotic stress including human land use is known to stress bat health and drive them closer to potential transitional hosts, a process implicated in spillover of other viruses (2,3).
We report a coronavirus in bats in Bangladesh that has high similarity to SARS-CoV-1 Tor2, isolated in 2003 from a febrile patient who had secondary exposure to a person who contracted coronavirus from an environmental source in southern China (5). The virus detected in Bandarban, Bangladesh, and sequenced and analyzed in this study shares identity with Tor2, except in the NSP2 and RBD genomic regions. The synthetically expressed RBD shows moderate binding affinity to ACE2 receptors of nearby species, suggesting potential for infection of co-occurring taxa within the host range. Additional study is needed to elucidate what drives host viral shedding and if spillovers are occurring that pose a public health risk. Describing the genetic diversity and transmission potential of this and other potentially zoonotic pathogens can aid in identifying sources and risk of future emerging spillovers.
Dr. Bradburne is a biologist and project manager at The Johns Hopkins University Applied Physics Laboratory. His research interests include applying sequencing and bioinformatics to viral and bacterial detection and diagnostics. Dr. Islam is a veterinarian working at the Infectious Diseases Division of the icddh,b. His research interests include zoonotic diseases.
Acknowledgments
We thank April Manzella for Biosafety Level 4 laboratory support, Mike Lee for bioinformatics support, and Robert Bull for support and encouragement throughout this study. We also thank Emma Spence, Sara LaTrielle, Manuel Ruiz-Aravena, Agnieszka Rynda-Apple, and Monica Hall for administrative and logistic support.
C.M., R.K.P., and E.S.G. were supported by the Defense Advanced Research Projects Agency’s PREventing EMerging Pathogenic Threats program (cooperative agreement no. D18AC00031). R.K.P. (grant no. DEB-1716698) and J.L., D.J., and R.K.P. (grant nos. EF-2133763 and EF-2231624) were supported by the National Science Foundation. This work was funded in part under agreement no. HSHQDC-15-C-00064 awarded to Battelle National Biodefense Institute by the US Department of Homeland Security (DHS) Science and Technology Directorate for the management and operation of the National Biodefense Analysis and Countermeasures Center, a federally funded research and development center.
The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of DHS or the US government. DHS does not endorse any products or commercial services mentioned in this presentation. In no event shall DHS, Battelle National Biodefense Institute, or National Biodefense Analysis and Countermeasures Center have any responsibility or liability for any use, misuse, inability to use, or reliance upon the information contained herein. In addition, no warranty of fitness for a particular purpose, merchantability, accuracy, or adequacy is provided regarding the contents of this document.
This manuscript has been coauthored by UT-Battelle, LLC, under contract no. DE-AC05-00OR22725 with the US Department of Energy (DOE). The publisher, by accepting the article for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
References
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, et al. Pathways to zoonotic spillover. Nat Rev Microbiol. 2017;15:502–10. DOIPubMedGoogle Scholar
- Eby P, Peel AJ, Hoegh A, Madden W, Giles JR, Hudson PJ, et al. Pathogen spillover driven by rapid changes in bat ecology. Nature. 2023;613:340–4. DOIPubMedGoogle Scholar
- Cashman M, Vergara VGM, Lagergren JH, Lane M, Merlet J, Atkinson M, et al. Longitudinal effects on plant species involved in agriculture and pandemic emergence undergoing changes in abiotic stress. In: Proceedings of the Platform for Advanced Scientific Computing conference; 2023 Jun 26–28; Davos, Switzerland. New York: ACM; 2023.
- Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019;20:257. DOIPubMedGoogle Scholar
- Marra MA, Jones SJM, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, et al. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–404. DOIPubMedGoogle Scholar
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–66. DOIPubMedGoogle Scholar
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020;37:1530–4. DOIPubMedGoogle Scholar
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14:587–9. DOIPubMedGoogle Scholar
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol. 2018;35:518–22. DOIPubMedGoogle Scholar
- Samson S, Lord É, Makarenkov V. SimPlot++: a Python application for representing sequence similarity and detecting recombination. Bioinformatics. 2022;38:3118–20. DOIPubMedGoogle Scholar
- Ruan YJ, Wei CL, Ee AL, Vega VB, Thoreau H, Su ST, et al. Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet. 2003;361:1779–85. DOIPubMedGoogle Scholar
- Song Y, Rodgers VGJ, Schultz JS, Liao J. Protein interaction affinity determination by quantitative FRET technology. Biotechnol Bioeng. 2012;109:2875–83. DOIPubMedGoogle Scholar
- Akhter T, Khan MMH, Nath S, Hasan S, Ahmed T. Photographic evidences of Pygmy white-toothed shrew Suncus etruscus in Bangladesh. Bangladesh J Zool. 52:337–40. DOIGoogle Scholar
- Kim Y, Son K, Kim Y-S, Lee S-Y, Jheong W, Oem J-K. Complete genome analysis of a SARS-like bat coronavirus identified in the Republic of Korea. Virus Genes. 2019;55:545–9. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: July 16, 2025
1These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 8—August 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Addresses for correspondence: Chris Bradburne, Johns Hopkins University Applied Physics Laboratory, 11100 Johns Hopkins Rd, Laurel, MD 20723-6099, USA
Top