Volume 29, Number 1—January 2023
Dispatch
Efficient Inactivation of Monkeypox Virus by World Health Organization‒Recommended Hand Rub Formulations and Alcohols
Figure 2
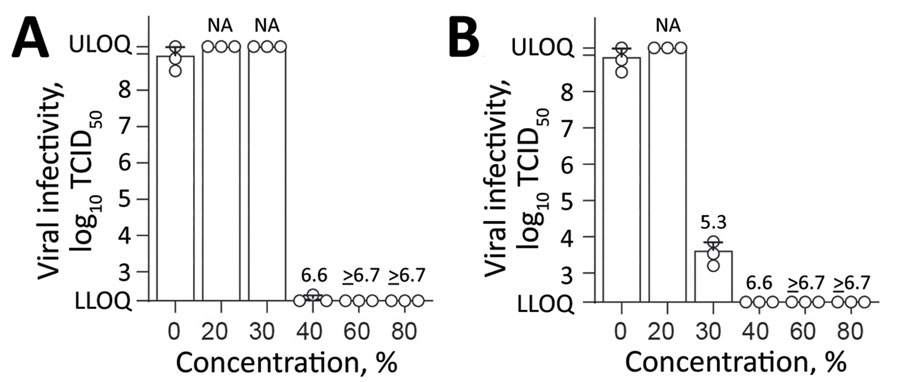
Figure 2. Effect of commercially available alcohols in inactivating monkeypox virus. A) Results for ethanol. B) Results for 2-propanol. Means of 3 independent experiments with SDs (error bars) are shown. Reduction factors are included above the bars. Biocide concentrations ranged from 0% to 80% with an exposure time of 30 s. Viral titers are displayed as TCID50/mL values. LLOQ, lower limit of quantification (1.58 × 102 TCID50/mL); NA, not applicable; TCID50, 50% tissue culture infectious dose; ULOQ, upper limit of quantification (1.58 × 109 TCID50/mL).
Page created: November 09, 2022
Page updated: December 22, 2022
Page reviewed: December 22, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.