Volume 29, Number 10—October 2023
Research
Characteristics of and Deaths among 333 Persons with Tuberculosis and COVID-19 in Cross-Sectional Sample from 25 Jurisdictions, United States
Cite This Article
Citation for Media
Abstract
Little is known about co-occurring tuberculosis (TB) and COVID-19 in low TB incidence settings. We obtained a cross-section of 333 persons in the United States co-diagnosed with TB and COVID-19 within 180 days and compared them to 4,433 persons with TB only in 2020 and 18,898 persons with TB during 2017‒2019. Across both comparison groups, a higher proportion of persons with TB–COVID-19 were Hispanic, were long-term care facility residents, and had diabetes. When adjusted for age, underlying conditions, and TB severity, COVID-19 co-infection was not statistically associated with death compared with TB infection only in 2020 (adjusted prevalence ratio 1.0 [95% CI 0.8‒1.4]). Among TB–COVID-19 patients, death was associated with a shorter interval between TB and COVID-19 diagnoses, older age, and being immunocompromised (non-HIV). TB–COVID-19 deaths in the United States appear to be concentrated in subgroups sharing characteristics known to increase risk for death from either disease alone.
Tuberculosis (TB) and COVID-19 were leading infectious causes of illness and death globally in 2020. In the United States, >17 million COVID-19 cases and ≈7,000 TB cases were reported in 2020 (1). Both TB and COVID-19 are primarily respiratory illnesses with overlapping signs and symptoms, and the Centers for Disease Control and Prevention (CDC) lists TB as a medical risk factor for COVID-19‒related disease severity and death (2). Few population-based reports of outcomes for persons with both TB and COVID-19 have been reported (3,4), and the definition of TB and COVID-19 co-diagnosis differs across studies. Those reports, and meta-analyses incorporating case reports and small observational series (5–8), have demonstrated higher mortality rates for persons with TB and COVID-19 compared with COVID-19 alone. Little has been published to adequately assess COVID-19 as a risk factor for poor TB outcomes. Furthermore, limited information is available from low TB incidence populations, including the United States. An analysis from California showed increased mortality rates for persons with TB and COVID-19 compared with TB only reported before the COVID-19 pandemic, particularly when TB and COVID-19 were diagnosed in close succession (9). That analysis indicated groups of persons who were disproportionately affected by both diseases, including Hispanic persons and those with diabetes or living in low health equity neighborhoods according to the California Healthy Places Index (10).
The COVID-19 pandemic also affected TB epidemiology and program management across epidemiologic contexts (11). In the United States, reported TB incidence declined ≈20% in 2020 compared with 2019 (12). Limited information suggests that some persons with TB in the United States may have had more clinically severe disease in 2020 than before the COVID-19 pandemic (13), and TB diagnoses may have been delayed (14). We aimed to describe demographic, social, and clinical characteristics of persons with TB and COVID-19 in the United States, including risk for death, and to identify populations who may benefit most from integrated interventions.
Design and Population
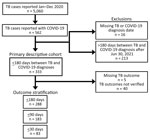
We established a voluntary collaboration of US health jurisdictions to obtain a cross-sectional sample of persons with TB and COVID-19 diagnosed within 180 days (hereafter TB–COVID-19). We used the population-based National Tuberculosis Surveillance System (NTSS) for cases reported during 2017‒2021 for standardized demographic, social, underlying conditions, and TB-specific diagnosis and treatment variables (15). Each jurisdiction captured a subset of the standardized data elements from the National Notifiable Disease Surveillance System for COVID-19 cases (16) and contributed them to this project. The core set of COVID-19 and TB surveillance data elements were consistent across jurisdictions. We included all persons with COVID-19 meeting the public health surveillance case definition for confirmed or probable COVID-19 (17) reported during January 1, 2020–June 30, 2021, who were also persons with TB reported in 2020 (i.e., persons with TB–COVID-19). Although the methods used by participating jurisdictions to identify persons with TB–COVID-19 varied (Appendix Table 1), each jurisdiction systematically identified their residents with co-diagnoses of TB and COVID-19 using personal identifiers. Of 26 participating jurisdictions, 11 (42.3%) used a software algorithm that included name and date of birth to match persons (several also included various combinations of sex, race/ethnicity, and place of residence), 8 (30.8%) had integrated surveillance systems (i.e., a given individual’s TB and COVID-19 diagnoses were already linked to a single record), and 1 (3.8%) with an integrated surveillance system also performed a name-based software match (Appendix Table 1). Directly identifiable information in the surveillance registries was retained by participating jurisdictions and not shared with investigators in other jurisdictions or with CDC.
Each jurisdiction securely transmitted data on persons with TB–COVID-19 to CDC. We then excluded persons with TB–COVID-19 with unknown TB or COVID-19 diagnosis dates and for which diagnoses occurred >180 days apart, regardless of which disease was diagnosed first (Figure 1). For analysis of TB treatment outcomes, we excluded jurisdictions with incomplete TB case outcome data. To identify characteristics of persons with TB–COVID-19 that differed from persons with TB only, we compared them to characteristics of persons with TB reported in 2020 without COVID-19 (i.e., 2020 TB-only) and TB reported during the 3 most recent pre–COVID-19 years, 2017‒2019 (i.e., 2017‒2019 TB-only).
Data Elements
NTSS data included demographic, social, and clinical characteristics, and TB diagnosis and treatment outcomes. We used a composite all-cause death outcome that included TB diagnosed after death, death occurring before or during TB treatment, and death recorded on the COVID-19 case report form. We defined the TB diagnosis date as the earliest among positive smear or tissue collection, positive nucleic acid amplification test result, first culture specimen collected for phenotypic drug-sensitivity testing, or TB treatment start date. For the COVID-19 diagnosis date, we used the date of specimen collection of the first positive nucleic acid amplification test or antigen test. We defined persons with disseminated TB as having meningeal or miliary disease, both pulmonary and extrapulmonary disease, or having a positive culture for Mycobacterium tuberculosis complex from blood.
Analytic Methods
We compared characteristics of persons with TB–COVID-19 with those of persons with 2020 TB-only and 2017‒2019 TB-only, calculating statistically significant differences of bivariate frequencies by using the Mantel-Haenszel χ2 test (or Fisher exact test for small cell counts) with Bonferroni correction for multiple comparisons. We also calculated Clopper–Pearson binomial 95% CIs for some frequencies. For continuous variables, we assessed differences in parametric means by using t-tests. We used the Wilcoxon rank-sum test to compare nonparametric continuous variables. We calculated prevalence ratios (PRs) and 95% CIs by using log-binomial multivariable regression employing backward selection in logistic regression models to identify statistically significant (α = 0.05) variables for inclusion in the log-binomial models. The final models included all variables reaching statistical significance and the COVID-19 co-diagnosis status as the exposure of interest. We did not assess interaction terms in multivariable models. Rather than exclude persons with missing covariate data, we classified missing values as unknown and retained them in the models. We stratified outcomes by the proximity in timing of TB and COVID-19 diagnoses (i.e., within 30, 90, and 180 days) and fit independent log-binomial models to each time interval.
Ethics Considerations
This activity was determined to meet the requirements of public health surveillance as defined in 45 CFR 46.102(l) (2). Informed consent was not required because the project was classified by CDC as nonresearch. Although most participating jurisdictions relied on the CDC project determination, some independently classified the activity as nonresearch.
TB–COVID-19 Analytic Population
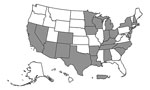
The 26 participating jurisdictions accounted for 62.9% of US TB cases in 2020 and 67.0% of the 2020 US population (18). The number of all TB cases reported in 2020 per participating jurisdiction ranged from 10 to 1,703: 12 jurisdictions (46.2%) reported <75 cases, 8 (30.8%) reported 75‒149 cases, and 6 (23.1%) reported >150 cases. Participating jurisdictions reported ≈64% of the ≈46,353,000 COVID-19 cases reported in US states and territories reported during the observation period (1). Jurisdictions using more robust methods (i.e., a software algorithm or an integrated surveillance system) to identify persons with TB–COVID-19 (Appendix Table 1) accounted for 91.7% of the TB cases among the 26 participating jurisdictions.
One of 26 jurisdictions (North Dakota) did not identify persons with TB–COVID-19 meeting our criteria, and so we excluded numerator and denominator data for this jurisdiction from statistical analyses. The remaining 25 jurisdictions (23 US states; New York, NY; and Puerto Rico) identified 333 persons with TB–COVID-19 meeting study criteria (Figures 1, 2). The number of persons with TB–COVID-19 identified per health jurisdiction ranged from 1 to 114 (Figure 2). The median age of persons with TB–COVID-19 was 55 years (interquartile range 35‒69 years); 204 (61.3%) were male and 129 (38.7%) female, and 264 (79.3%) were non–US-born (Table 1). Six (1.8%) persons were co-diagnosed with TB and COVID-19 on the same date, and 65 (19.5%) persons were co-diagnosed within 14 days (Figure 3). Of the 327 TB–COVID-19 cases diagnosed >1 day apart, 204 (62.4%) had TB diagnosed before COVID-19.
TB–COVID-19 Demographics Compared with 2020 TB-only and 2017‒2019 TB-only
We did not find statistically significant (95% CI with Bonferroni correction) bivariate differences in persons with TB–COVID-19 relative to the 2020 TB-only and 2017‒2019 TB-only comparison groups for sex, residence in a correctional facility, homelessness, or excessive alcohol use (Table 1). The TB–COVID-19 group had a higher proportion of Hispanic persons compared with both of the reference groups (Table 1). Higher proportions of persons with TB–COVID-19 also were residents of long-term care facilities at TB diagnosis compared with both reference groups.
TB–COVID-19 Clinical Characteristics Compared with 2020 TB-only and 2017‒2019 TB-only
We did not find statistically significant differences, compared with either the 2020 TB-only or 2017‒2019 TB-only reference group, for the proportion of persons with TB–COVID-19 by the status of a previous episode of TB, HIV infection, TB disease site (i.e., pulmonary-only, extrapulmonary, or both sites), or TB disease dissemination (Table 1). Compared with both reference groups, persons with TB–COVID-19 had a higher rate of diabetes and end-stage renal disease.
Multivariable Comparison of TB–COVID-19 Characteristics Compared with 2020 TB-only and 2017‒2019 TB-only
In comparison to 2020 TB-only, persons with TB and COVID-19 diagnosed within 180 days were more likely to be in American Indian/Alaska Native (adjusted prevalence ratio [aPR] 5.3 [95% CI 2.1‒13.4]) and, with borderline statistical significance, Native Hawaiian/Other Pacific Islander (aPR 2.3 [95% CI 1.0‒5.3]) relative to non-Hispanic whites (Table 2). Persons with TB–COVID-19 also had a higher proportion of being non–US-born (aPR 1.5 [95% CI 1.1‒2.1]), residing in long-term care facilities at TB diagnosis (aPR 2.5 [95% CI 1.6‒4.0]), and having diabetes (aPR 1.6 [95% CI 1.3‒2.0]). When comparing persons with TB–COVID-19 to 2017‒2019 TB-only, those associations remained statistically significant; in addition, a higher proportion of persons with TB–COVID-19 also had end-stage renal disease (aPR 1.7 [95% CI 1.1‒2.7]) and, with borderline statistical significance, acid-fast bacilli sputum smear positivity (aPR 1.3 [95% CI 1.0‒1.6]) (Table 2).
Mortality Rates Compared with 2020 TB-only Patients
The occurrence of death at any time before or during TB treatment was 450/3,793 (11.9% [95% CI 10.8‒12.9]) for 2020 TB-only. This rate compares with 48/288 (16.7% [95% CI 12.6‒21.5]; unadjusted prevalence ratio [uPR] 1.4 [95% CI 1.1‒1.8]) for persons co-diagnosed with TB and COVID-19 within 180 days, 39/183 (21.3% [95% CI 15.6‒28.0]; uPR 1.8 [95% CI 1.3‒2.5]) for those co-diagnosed within 90 days, and 17/83 (20.5% [95% CI 12.4‒30.8]; uPR 1.7 [95% CI 1.1‒2.7]) for those co-diagnosed within 30 days (Figure 4). After adjustment for age, comorbidities, and markers of TB disease severity, COVID-19 did not retain significance as an independent risk factor for all-cause mortality in persons with TB disease (Appendix Table 2). Significant cofactors were age ≥45 years, HIV infection (aPR 2.1 [95% CI 1.3‒3.5]), end-stage renal disease (aPR 1.8 [95% CI 1.4‒2.4]), TB disease dissemination (aPR 1.5 [95% CI 1.1‒1.9]), and sputum smear positivity for acid-fast bacilli (aPR 1.4 [95% CI 1.1‒1.8]). We observed similar associations when persons had TB and COVID-19 diagnosed within shorter intervals (i.e., 30 and 90 days) (Appendix Table 2).
Risk Factors for Death among TB–COVID-19 Patients
Among the 288 persons with TB–COVID-19 with known mortality outcome (86.5% of all 333 persons with TB–COVID-19), death was associated with both TB and COVID-19 co-diagnoses being made within 90 days (aPR 2.3 [95% CI 1.1‒4.8]), being immunocompromised (non-HIV) (aPR 2.7 [95% CI 1.1‒6.4]), and age (Table 3). The adjusted risk for death increased with increasing age compared with <44 years: 45‒64 years, aPR 5.6 (95% CI 1.6‒19.8); 65‒74 years, aPR 8.6 (95% CI 2.4‒31.3); 75‒84 years, aPR 12.6 (95% CI 3.5‒45.7); and >85 years, aPR 25.0 (95% CI 6.9‒91.1).
We report a large cohort of persons with TB–COVID-19 from a low TB–incidence setting (the United States) during the COVID-19 pandemic. Persons with TB and COVID-19 had overlapping sociodemographic and medical risk profiles known to be associated with each disease, including long-term care residence, diabetes, and end-stage renal disease. The frequency of death for persons with TB–COVID-19 was higher than persons with TB-only and depended on a shorter interval between TB and COVID-19 diagnoses (1 in 5 persons who had TB–COVID-19 co-diagnosed within 30 days died). However, COVID-19 was not independently associated with death among persons diagnosed with TB within 180 days when adjusted for age, underlying conditions, and TB disease severity, compared with those with 2020 TB-only patients. Among persons with TB–COVID-19, the timing of TB and COVID-19 co-diagnoses (i.e., within 90 days) remained a predictor of death, along with increasing age and being immunocompromised (non-HIV). Another analysis from California demonstrated an age-adjusted mortality rate ratio of 1.3 (95% CI 0.7‒2.5) for deaths among TB–COVID-19 patients compared with 2017‒2019 TB-only patients (9). That analysis did not adjust for underlying conditions (9). Increased mortality rates for persons with TB–COVID-19 has been repeatedly demonstrated in other settings when compared with persons with COVID-19 only (5–8). Other studies have demonstrated more severe COVID-19 disease classification among persons with TB–COVID-19 compared with persons diagnosed with COVID-19 without TB (19). However, few population-based studies have evaluated COVID-19 as a risk factor for all-cause mortality among persons with TB while adjusting for age, underlying conditions, and other potential confounders.
The baseline mortality rate for persons with TB was ≈9% annually in the United States in 2017 and 2018 (18). In our study, ≈17% of persons diagnosed with TB and COVID-19 within 180 days died. Nonetheless, in multivariable analysis corrected for age and underlying conditions, COVID-19 was not an independent predictor of death among persons with TB diagnosed within 180 days. Those findings suggest that poor outcomes for persons with TB–COVID-19 may be driven by the overlapping sociodemographic and medical risk factors common to each TB and COVID-19 that already place persons with TB at risk for death with TB, rather than the effect of COVID-19 coinfection alone. Compared with countries that have high TB prevalence, TB disease in the United States and other low TB incidence countries is more concentrated in older persons who have underlying conditions such as diabetes and renal disease (18). The timing of TB and COVID-19 co-diagnoses and its association with TB mortality warrants more investigation, given that our model demonstrated an association between a smaller diagnostic interval (90 days) and death among persons with TB–COVID-19. In addition to biological mechanisms to explain the association, persons with more severe COVID-19 may have been more likely to receive chest imaging and additional diagnostic testing to reveal concurrent TB.
Delayed TB diagnoses could have led to more severe TB disease at clinical evaluation in our analysis population. Another study from a low TB incidence setting showed a higher proportion of positive microscopic examinations during the COVID-19 pandemic compared with historical trends (20), similar to the observation in this US cohort of persons with TB–COVID-19. This finding suggests longer delays until TB diagnosis during the COVID-19 pandemic. The timing of TB diagnosis after COVID-19 (a substantial proportion had TB diagnosed within 14 days after COVID-19) could also reflect delayed TB diagnoses, suggesting that COVID-19 could have brought persons with undiagnosed TB into care.
TB program participation was nonrandom, which limits the representativeness of results to the entire United States, perhaps especially related to race and ethnicity. Nonetheless, the cohort represented a cross-section of US jurisdictions with varying TB prevalence. An important distinction in comparison with other studies is that we were unable to compare outcomes for persons with TB–COVID-19 with those for persons with COVID-19 only. Other limitations are that the completeness of COVID-19 case reporting may have differed by jurisdiction and phase of the epidemic. Missing data may have lessened the accuracy of some descriptive characteristics; missing death dates precluded hazards analyses of time to death. Longitudinal case management for persons on TB treatment probably captured most, but potentially not all, deaths among persons with TB. Our definition for disseminated TB is intended to capture most cases resulting from hematogenous spread that might be associated with delayed diagnoses or poor outcomes. It may not reflect all disseminated TB characterized by isolated extrapulmonary lymphadenitis or TB misclassified because of incomplete tissue sampling. The Bonferroni correction may have raised the risk for type II error in bivariate comparisons, and the small number of persons with TB–COVID-19 and having sociodemographic characteristics potentially influencing outcomes (e.g., experiencing homelessness) limited our ability to describe them. Strengths include the high completeness of sociodemographic data available in NTSS (15). Still, some underlying conditions strongly associated with poor COVID-19 outcomes (e.g., cardiovascular disease and obesity) were not available.
In conclusion, this analysis of a US cohort of persons with TB–COVID-19 suggests deaths among persons with TB–COVID-19 in the United States is concentrated in subgroups having shared characteristics known to increase risk for death with either disease alone. Timely consideration for TB disease among persons with COVID-19 and TB risk factors should be reinforced. Because death was associated with shorter intervals between co-diagnoses, prioritizing additional early medical interventions for persons with concurrent disease processes who are at highest risk for death might improve outcomes. COVID-19 patients with severe disease may be given immunomodulating treatments that could reactivate latent TB infection. Therefore, COVID-19 patients with risk factors for TB infection could be considered for screening and treatment of latent TB infection. Last, integration of screening for TB infection (risk factor review and serum interferon gamma release assays testing) with community COVID-19 prevention efforts among subpopulations with shared risk profiles, as has been done for persons at increased risk for COVID-19 and diabetes (21), may expand high-yield opportunities to prevent TB.
Dr. Nabity is a medical officer for CDC’s Field Services Branch, Division of TB Elimination, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, currently assigned to the California Department of Public Health Tuberculosis Control Branch. His primary research interests include the epidemiology of infectious diseases, including TB and COVID-19.
Acknowledgments
We thank John A. Jereb, Susan McElhany, and Sabrina Nabinger.
Author contributions: All authors contributed to data interpretation and review and approval of the final manuscript. S.A.N., S.M.M., N.D.G., S.R.S., E.T., S.P., J.L.S., L.G., K.G.T., M.N., D.H.W., and S.H.W. conceived of the study design and analytic plan. The National Tuberculosis Controllers Association/CDC TB-COVID-19 Collaboration collected data. S.M.M. and S.P. curated and analyzed the data. S.A.N., S.M.M., and N.D.G. drafted the original manuscript. S.H.W., D.H.W., N.D.G., and S.A.N. coordinated the project.
National Tuberculosis Controllers Association/CDC TB-COVID-19 Collaboration Members: Alabama Department of Public Health: Claire Payne; Arkansas Department of Health: Leonard Ntaate Mukasa, Virginia Maturino, Sandra E. Chai, Naveen Patil; California Department of Public Health: Emily Han, Pennan M. Barry, Seema Jain, Jennifer Flood; Colorado Department of Public Health and Environment: Juli Bettridge; Indiana Department of Health: Biak Chinpar, Sarah Bennett, Petia Boykova; Iowa Department of Health and Human Services: Allan Lynch; Kentucky Department of Public Health: Charles H. Rhea; Louisiana Department of Health: Andrew Smith, Kathryn Yoo; Massachusetts Department of Public Health: Andrew Tibbs, Kavita Gadani; Minnesota Department of Health: Katie Stinebaugh; New Hampshire Department of Health and Human Services: Carolyn R. Fredette, Elizabeth A. Talbot, Darlene Morse; New Jersey Department of Health: Julianna Wisniewski; New Mexico Department of Health: Brenda Montoya Denison; New York State Department of Health: Cheryl H. Kearns, Jamie Sommer; New York City Department of Health and Mental Hygiene: Soogyum Kim, Lisa Trieu; North Carolina Department of Health and Human Services: Daniela Ingram, Jennifer B. Wheeler; North Dakota Health & Human Services: Saurav Dahal; Ohio Department of Health: Sarah Mitchell, Sara Stokes; Puerto Rico Department of Health: Jose Calderon; South Carolina Department of Health & Environmental Control: Jesse S. Ellis; Tennessee Department of Health: Robert J. Cummins, Ben Katz; Texas Department of State Health Services: Anette Costa; Wisconsin Department of Health Services: Claire Leback; NTCA Working Group Members: Aakriti Pandita (University of Colorado), Matthew Dory (Maryland Department of Health), Maureen Murphy-Weiss (Columbus Public Health); CDC: Terence Chorba, Adam Langer, Farah Parvez, Benjamin Silk
References
- Johns Hopkins School of Public Health. Coronavirus Resource Center: cumulative cases. 2022 [cited 2022 Sep 23]. https://coronavirus.jhu.edu/data/cumulative-cases
- Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. 2022 [cited 2022 Sep 22]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
- Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond). 2020;52:902–7. DOIPubMedGoogle Scholar
- Boulle A, Davies M-A, Hussey H, Ismail M, Morden E, Vundle Z, et al. Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa. Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73:e2005–15. DOIGoogle Scholar
- Wang Y, Feng R, Xu J, Hou H, Feng H, Yang H. An updated meta-analysis on the association between tuberculosis and COVID-19 severity and mortality. J Med Virol. 2021;93:5682–6. DOIPubMedGoogle Scholar
- Aggarwal AN, Agarwal R, Dhooria S, Prasad KT, Sehgal IS, Muthu V. Active pulmonary tuberculosis and coronavirus disease 2019: A systematic review and meta-analysis. PLoS One. 2021;16:
e0259006 . DOIPubMedGoogle Scholar - Jindal R, Gupta M, Khan FR, Chaudhry G. Prevalence of co-morbidities and its association with mortality in Indian patients with COVID-19: A meta-analysis. Indian J Anaesth. 2022;66:399–418. DOIPubMedGoogle Scholar
- Wang Q, Guo S, Wei X, Dong Q, Xu N, Li H, et al. Global prevalence, treatment and outcome of tuberculosis and COVID-19 coinfection: a systematic review and meta-analysis (from November 2019 to March 2021). BMJ Open. 2022;12:
e059396 . DOIPubMedGoogle Scholar - Nabity SA, Han E, Lowenthal P, Henry H, Okoye N, Chakrabarty M, et al. Sociodemographic characteristics, comorbidities, and mortality among persons diagnosed with tuberculosis and COVID-19 in close succession in California, 2020. JAMA Netw Open. 2021;4:
e2136853 . DOIPubMedGoogle Scholar - Public Health Alliance of Southern California. The California Healthy Places Index. 2018 [cited 2021 Mar 12]. https://www.healthyplacesindex.org
- Dheda K, Perumal T, Moultrie H, Perumal R, Esmail A, Scott AJ, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. 2022;10:603–22. DOIPubMedGoogle Scholar
- Deutsch-Feldman M, Pratt RH, Price SF, Tsang CA, Self JL. Tuberculosis - United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:409–14. DOIPubMedGoogle Scholar
- Louie JK, Agraz-Lara R, Romo L, Crespin F, Chen L, Graves S. Tuberculosis-associated hospitalizations and deaths after COVID-19 shelter-in-place, San Francisco, California, USA. Emerg Infect Dis. 2021;27:2227–9. DOIPubMedGoogle Scholar
- Narita M, Hatt G, Gardner Toren K, Vuong K, Pecha M, Jereb JA, et al. Delayed tuberculosis diagnoses during the coronavirus disease 2019 (COVID-19) pandemic in 2020—King County, Washington. Clin Infect Dis. 2021;73(Suppl 1):S74–6. DOIPubMedGoogle Scholar
- Yelk Woodruff RS, Pratt RH, Armstrong LR. The US National Tuberculosis Surveillance System: a descriptive assessment of the completeness and consistency of data reported from 2008 to 2012. JMIR Public Health Surveill. 2015;1:
e15 . DOIPubMedGoogle Scholar - Council of State and Territorial Epidemiologists. Update to the standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). 2020 [cited 2023 Jan 9]. https://www.cste.org/resource/resmgr/ps/positionstatement2020/Interim-20-ID-02_COVID-19.pdf
- Centers for Disease Control and Prevention. COVID-19 2020 interim case definition, approved August 5, 2020. 2020 [cited 2022 Sep 23]. https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2020-08-05
- Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2020. 2021 [cited 2022 Sep 23]. https://www.cdc.gov/tb/statistics/reports/2020/default.htm
- Lucien MAB, Pierre K, Jean-Denis G, Rigodon J, Worrell CM, Couture A, et al. Epidemiology and risk factors related to severity of clinical manifestations of COVID-19 in outpatients: A retrospective study in Haiti. PLoS One. 2022;17:
e0274760 . DOIPubMedGoogle Scholar - Marchese V, Formenti B, Marchese L, Gregori N, Gardini G, Russo G, et al. COVID-19 effect on TB presentation and outcome. Int J Tuberc Lung Dis. 2022;26:375–7. DOIPubMedGoogle Scholar
- Chapman LAC, Shukla P, Rodríguez-Barraquer I, Shete PB, León TM, Bibbins-Domingo K, et al. Risk factor targeting for vaccine prioritization during the COVID-19 pandemic. Sci Rep. 2022;12:3055. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: August 30, 2023
1Group members are listed at the end of this article.
Table of Contents – Volume 29, Number 10—October 2023
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Scott Nabity, California Department of Public Health, 850 Marina Bay Pkwy, Bldg P Fl 2, Richmond, CA 94804-6403, USA
Top