Volume 29, Number 2—February 2023
Research Letter
Serologic Evidence of Orientia Infection among Rural Population, Cauca Department, Colombia
Abstract
We assessed serum samples collected in Cauca Department, Colombia, from 486 persons for Orientia seroreactivity. Overall, 13.8% showed reactive IgG by indirect immunofluorescence antibody assay and ELISA. Of those samples, 30% (20/67) were confirmed to be positive by Western blot, showing >1 reactive band to Orientia 56-kD or 47-kD antigens.
Scrub typhus, caused by species in the genus Orientia, is a reemerging mite-borne rickettsiosis and a major cause of acute undifferentiated febrile illness (AUFI) (1). Classically, scrub typhus was believed to be strictly endemic to the so-called tsutsugamushi triangle, which ranges from southeastern Siberia in the North to the Kamchatka Peninsula in the East, northern Australia in the South, and Pakistan in the West (1). However, scrub typhus outside the tsutsugamushi triangle was suggested 70 years ago because seropositivity to O. tsutsugamushi was found among persons from several countries in Africa (1).
In 2006, a confirmed case of scrub typhus outside the classical disease-endemic region was described in a traveler who visited Dubai (United Arab Emirates); the case was caused by a novel species, Candidatus Orientia chuto (2). More recently, in Latin America, autochthonous cases of scrub typhus have been reported in Chile and attributed to a new Orientia species, Candidatus Orientia chiloensis, associated with Herpetacarus spp. mites, which have been recognized as novel vectors of scrub typhus in this newly recognized disease-endemic region (3,4).
In addition to the aforementioned studies in Chile, serologic surveys have been performed among military personnel stationed in the Peruvian Amazon and Honduras, demonstrating exposure to Orientia, as well as a possible role for this bacterium in the etiology of AUFI (5,6). Thus, to explore new geographic regions that might have Orientia circulation, we report serologic evidence of this rickettsial agent in Colombia.
We screened 486 serum samples collected in 4 municipalities in the Cauca Department, Colombia, during 2017 and used in a previous study (7) for Orientia seroreactivity. Cauca Department is located in southwestern Colombia, mainly in the Andean and Pacific regions plus a tiny part in the Amazonian region. The climate is warm and humid on the western slope; warm and semiarid toward the Patía basin; and temperate humid to semihumid centrally, with cold climates recorded on both sides of the Popayán (capital city) Plateau.
We tested for antibodies reactive to O. tsutsugamushi by indirect immunofluorescence antibody (IFA) assay and ELISA (Appendix). To further confirm the specificity of the reactive samples, we performed a Western blot assay (Appendix).
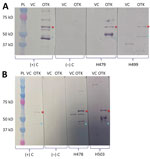
Of 486 serum samples, 6 (1%) were seropositive for Orientia by IFA with an endpoint titer of 1:128 to 1:1,024, and 62 (13%) yielded positive results by ELISA (optical density cutoff >0.37). Only 1 sample was positive by both methods. Overall, 67 (13.8%) serum samples were positive by >1 screening method. Testing of 67 of the seropositive samples by Western blot showed that 20 (30%) were reactive with >1 O. tsutsugamushi band. A total of 8 samples were reactive to 56-kD, 5 to 47-kD, and 7 to both (Table; Figure).
Our results show serologic evidence of Orientia exposure among the rural population of 4 municipalities from Cauca Department, Colombia. This evidence suggested circulation of this rickettsial agent in this region.
Using 2 serologic techniques (IFA and ELISA), we found different seropositivity rates (1% for IFA and 13% for ELISA) for the same serum samples. Similar discordance has been observed for persons from Sao Tome and Principe (8). The difference in seropositivity rates between methods might be caused by the antigens used because our IFA used only antigens of the Karp strain, which could fail to detect antibodies stimulated by organisms of other serotypes and species. Moreover, some autochthonous cases of scrub typhus in Chile have also shown discordant serologic results (reactive IgG by IFA/negative IgG by ELISA, and vice versa) (9,10).
As briefly mentioned, the use of ELISA to detect IgG reactive against Orientia showed reactivity in 5.3% of convalescent-phase serum samples from febrile patients in the region of Iquitos (Peruvian Amazon) (5) and in 5.6% of US military personnel stationed at least 6 months in Honduras (6). Our study found 13% seropositivity by using the same serologic approach (IgG ELISA), which is greater than results obtained in Perú and Honduras. In addition to ELISA and IFA screening methods, we used a confirmatory test (Western blot), which demonstrated reactivity to >1 immunodominant protein (56 kD and 47 kD) of Orientia in 30% of the tested samples, which were positive by >1 screening method.
In conclusion, we report serologic evidence of Orientia in Colombia, supporting that these bacteria are probably widely distributed in Latin America. Our study also supports emerging evidence that Orientia spp. is no longer restricted to the tsutsugamushi triangle. Prospective studies to examine acute-phase and convalescent-phase serum samples and isolation of the agent in those with AUFI in Colombia and other countries in Latin America should be undertaken. In addition, scrub typhus should be considered as a potential diagnosis by physicians and public health officials when an eschar-associated rickettsiosis is suspected in this region.
Dr. Faccini-Martínez is a physician‒scientist at Fundación Universitaria de Ciencias de la Salud, Bogotá, Colombia. His primary research interests include zoonotic and vector-borne diseases.
Acknowledgments
We thank Nicole Mendell, Donald H. Bouyer and Patricia Valdes for providing in-house slides coated and fixed with whole-cell antigens of O. tsutsugamushi Karp strain, and whole Vero cells extracts and whole-cell antigens of O. tsutsugamushi Karp strain for Western blot assay.
This study was supported by COLCIENCIAS (project no. 120374455209, Colombia). At the time of this study, Á.A.F.-M. was supported by the Fogarty International Center and the National Institute of Allergy and Infectious Diseases, National Institutes of Health under grant D43 TW010331.
References
- Richards AL, Jiang J. Scrub typhus: historic perspective and current status of the worldwide presence of Orientia species. Trop Med Infect Dis. 2020;5:49. DOIPubMedGoogle Scholar
- Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404–9. DOIPubMedGoogle Scholar
- Abarca K, Martínez-Valdebenito C, Angulo J, Jiang J, Farris CM, Richards AL, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148–56. DOIPubMedGoogle Scholar
- Weitzel T, Silva-de la Fuente MC, Martínez-Valdebenito C, Stekolnikov AA, Pérez C, Pérez R, et al. Novel vector of scrub typhus in sub-Antarctic Chile: evidence from human exposure. Clin Infect Dis. 2022;74:1862–5. DOIPubMedGoogle Scholar
- Kocher C, Jiang J, Morrison AC, Castillo R, Leguia M, Loyola S, et al. Serologic evidence of scrub typhus in the Peruvian Amazon. Emerg Infect Dis. 2017;23:1389–91. DOIPubMedGoogle Scholar
- Chao CC, Zhang Z, Belinskaya T, Chen HW, Ching WM. Leptospirosis and rickettsial diseases sero-conversion surveillance among U.S. military personnel in Honduras. Mil Med. 2022;187:802–7. DOIPubMedGoogle Scholar
- Forero-Becerra E, Patel J, Martínez-Díaz HC, Betancourt-Ruiz P, Benavides E, Durán S, et al. Seroprevalence and genotypic analysis of Ehrlichia canis infection in dogs and humans in Cauca, Colombia. Am J Trop Med Hyg. 2021;104:1771–6. DOIPubMedGoogle Scholar
- Yen TY, Zhang Z, Chao CC, Ching WM, Shu PY, Tseng LF, et al. Serologic Evidence for Orientia Exposure in the Democratic Republic of Sao Tome and Principe. Vector Borne Zoonotic Dis. 2019;19:821–7. DOIPubMedGoogle Scholar
- Weitzel T, Dittrich S, López J, Phuklia W, Martinez-Valdebenito C, Velásquez K, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954–61. DOIPubMedGoogle Scholar
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, Jiang J, Richards AL, Abarca K. Scrub typhus in continental Chile, 2016–2018. Emerg Infect Dis. 2019;25:1214–7. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: January 16, 2023
Table of Contents – Volume 29, Number 2—February 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
David H. Walker, Department of Pathology, University of Texas Medical Branch, Galveston, TX 77555, USA
Top