Volume 29, Number 3—March 2023
Dispatch
Nosocomial Severe Fever with Thrombocytopenia Syndrome in Companion Animals, Japan, 2022
Cite This Article
Citation for Media
Abstract
In Japan, 2 cats that underwent surgery in a room where a sick dog had been euthanized became ill within 9 days of surgery. Severe fever with thrombocytopenia syndrome virus was detected in all 3 animals; nucleotide sequence identity was 100%. Suspected cause was an uncleaned pulse oximeter probe used for all patients.
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging and mostly fatal tickborne zoonosis in eastern Asia. The causative agent is Dabie bandavirus, of the family Phenuiviridae and genus Bandavirus, and is generally known as SFTS virus (SFTSV). In Japan, SFTS-related mortality rates are reported to be 27% among humans and 62% among domestic cats (1,2). Although dogs can become infected with SFTSV, the mortality rate is unclear because infection of healthy dogs tends be subclinical (3).
SFTSV is transmitted to humans and animals primarily through tick bites. However, nosocomial infection without a tick bite can occur via contact with blood and body fluids (4). Human-to-human transmission from an index patient to healthcare workers has been reported (4). Animal-to-human transmission from an index animal to veterinary personnel has also been reported (5,6). We report a nosocomial animal-to-animal transmission of SFTSV.
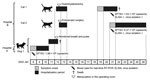
On January 8, 2022, a 13-year-old female dog (dog 1) with a high fever (39.9°C [reference range 38.0°C–39.0°C]) and anorexia was examined at animal hospital A (Table; Figure 1). The next day, dog 1 exhibited diarrhea and neurologic symptoms (unsteadiness and wandering). When the animal’s condition did not improve, on January 11, the dog was transferred to animal hospital B. On the basis of a high concentration of pancreas-specific lipase and pancreatic ultrasonography findings, veterinarians in animal hospital B diagnosed pancreatitis. Infectious disease was not suspected because the dog had no signs of a tick bite and had been vaccinated against most of the severe canine diseases in Japan. At 11:00 a.m. the next day, the dog was unresponsive to stimuli. The dog underwent tracheal intubation and mechanical ventilation, and a pulse oximeter probe was placed on the tongue. The dog did not respond to treatment and was euthanized and returned to the owner at approximately 3:00 p.m.
On January 12, at approximately 10 a.m., a healthy 7-month-old female domestic cat (cat 1) was hospitalized at animal hospital B for ovariohysterectomy (Figure 1). At approximately 4:00 p.m., the ovariohysterectomy was performed under anesthesia on the same operating table and with the same ventilator used for dog 1. Cat 1 was discharged in healthy condition the next day.
Also on January 12, at approximately 6 p.m., a 21-month-old male domestic cat (cat 2) was urgently hospitalized at animal hospital B for ingestion of a foreign body. Cat 2 underwent endoscopic surgery under anesthesia in the same operating room and was discharged in healthy condition the next day.
Cats 1 and 2 had no contact with dog 1 in the hospital. After surgery, the cats were kept in the same hospital room but in different cages and had no contact with each other. All 3 animals had different owners, and no contact before hospitalization was reported.
On January 19, a high fever (40.8°C [reference range 38.0°C–39.0°C]), vomiting, and inappetence developed in cat 1. Its condition worsened; on January 21, leukopenia and thrombocytopenia were confirmed (Table), and on January 22, the cat died. On January 21, high fever (39.8°C) with bilirubinuria developed in cat 2. Despite vomiting on January 22, cat 2 recovered by January 26. Both cats were kept indoors only, and neither had a history of a tick bite.
The director of animal hospital B suspected nosocomial infections because severe symptoms developed in the 2 cats that had undergone surgery on the same day. Serum samples from the cats were sent to the Center for Animal Disease Control, University of Miyazaki, where real-time reverse transcription PCR for SFTSV, feline calicivirus, and feline parvovirus was performed (9–11).
High copy numbers of SFTSV RNA were detected in both samples (cat 1 = 1.53 × 106 copies/mL; cat 2 = 6.37 × 106 copies/mL). Also confirmed by using double-antigen ELISA were IgG, IgM, or both against SFTSV nucleoprotein (absorbance at 405 nm) (cat 1 = 0.39; cat 2 = 2.92) (8). Blood collected from dog 1 on January 8 had been discarded in the medical waste box but was retrieved and sent to the Center for Animal Disease Control after results for the cats were confirmed. Although the blood had been kept at room temperature for >2 weeks, a high copy number of SFTSV RNA was detected (1.99 × 106 copies/mL). ELISA was not performed because the blood was in poor condition. For veterinary personnel, body temperature and real-time reverse transcription PCR were monitored daily by the Oita City Public Health Center, but SFTSV infection was not detected.
SFTSV isolation was performed by using serum from cats 1 and 2 and hemolyzed blood from dog 1. The virus isolation procedure has been previously described (12). SFTSV was isolated from both cats but not from the dog because of poor preservation of the dog sample. Next, the entire sequences of the SFTSV medium (M) segment from the animals were compared. The M segment encoding Gn and Gc glycoproteins is a more diverse segment than the small and large segments (13). Almost the entire sequence (SFTSV M segment, nt 9–3378) was successfully amplified and determined by using the reported primers (13) and submitted to the DNA Data Bank of Japan (accession no. LC705155-7). The virus sequences from the index dog and the 2 secondarily affected cats showed 100% homology (Figure 2). Furthermore, the sequences were most closely related (99.8%) to the SFTSV SPL105A Miyazaki 2013 strain (accession no. AB985315), which was obtained from a person with SFTS infection in an adjacent prefecture in 2013.
The operating room was a sanitary environment. The operating table was disinfected after each use; repeated use of contaminated instruments was prohibited; and all staff wore disposable gowns, masks, and gloves during operations. Although most medical instruments do not cause nosocomial infection, we determined that the pulse oximeter probe posed the highest risk for virus transmission between the dog and the cats because a disposable paper towel was placed between the probe and tongue, with saliva contaminating the probe, and the staff were unable to confirm whether the inner surface of the probe was wiped with hypochlorous acid between patients. A previous study detected high levels of viral RNA in the saliva of animals with SFTS (14,15). Because the same ventilator was used with the 3 animals reported here, aerosol transmission is another suspected source. Although the tracheal tubes and attached equipment were changed after each use, other parts (e.g., the breathing tube) were not changed and disinfected because infectious disease was not suspected.
We report molecular evidence of nosocomial transmission of SFTSV among companion animals in an animal hospital in Japan. Veterinary personnel should be aware of the risk that this emerging zoonotic disease poses for their safety as well as the safety of patients and clients. To prevent nosocomial infections, veterinary staff should be educated about basic infection prevention and control practices in animal hospitals.
Dr. Mekata is an associate professor with the Center for Animal Disease Control, University of Miyazaki, Miyazaki, Japan. His research interests are epidemiology, prevention measures, and the viral evolution of animal infectious diseases.
Acknowledgments
We thank the veterinarians and clients for permission to report this study. We also thank Mari Yamamoto and Saori Kusakari for their technical assistance.
This study was supported by the Japan Society for the Promotion of Science, KAKENHI (grant no. 21H02361).
References
- Kobayashi Y, Kato H, Yamagishi T, Shimada T, Matsui T, Yoshikawa T, et al.; SFTS Epidemiological Research Group Japan. Severe fever with thrombocytopenia syndrome, Japan, 2013–2017. Emerg Infect Dis. 2020;26:692–9. DOIPubMedGoogle Scholar
- Matsuu A, Momoi Y, Nishiguchi A, Noguchi K, Yabuki M, Hamakubo E, et al. Natural severe fever with thrombocytopenia syndrome virus infection in domestic cats in Japan. Vet Microbiol. 2019;236:
108346 . DOIPubMedGoogle Scholar - Park SC, Park JY, Choi JY, Oh B, Yang MS, Lee SY, et al. Experimental infection of dogs with severe fever with thrombocytopenia syndrome virus: Pathogenicity and potential for intraspecies transmission. Transbound Emerg Dis. 2022;69:3090–6. DOIPubMedGoogle Scholar
- Jung IY, Choi W, Kim J, Wang E, Park SW, Lee WJ, et al. Nosocomial person-to-person transmission of severe fever with thrombocytopenia syndrome. Clin Microbiol Infect. 2019;25:633.e1–4. DOIPubMedGoogle Scholar
- Yamanaka A, Kirino Y, Fujimoto S, Ueda N, Himeji D, Miura M, et al. Direct transmission of severe fever with thrombocytopenia syndrome virus from domestic cat to veterinary personnel. Emerg Infect Dis. 2020;26:2994–8. DOIPubMedGoogle Scholar
- Kida K, Matsuoka Y, Shimoda T, Matsuoka H, Yamada H, Saito T, et al. A case of cat-to-human transmission of severe fever with thrombocytopenia syndrome virus. Jpn J Infect Dis. 2019;72:356–8. DOIPubMedGoogle Scholar
- Umeki K, Yasuda A, Umekita K, Megumi R, Nomura H, Kawaguchi T, et al. Detection of anti-SFTSV nuclear protein antibody in the acute phase sera of patients using double-antigen ELISA and immunochromatography. J Virol Methods. 2020;285:
113942 . DOIPubMedGoogle Scholar - Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, Tominaga T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014;209:816–27. DOIPubMedGoogle Scholar
- Streck AF, Rüster D, Truyen U, Homeier T. An updated TaqMan real-time PCR for canine and feline parvoviruses. J Virol Methods. 2013;193:6–8. DOIPubMedGoogle Scholar
- Brunner C, Kanellos T, Meli ML, Sutton DJ, Gisler R, Gomes-Keller MA, et al. Antibody induction after combined application of an adjuvanted recombinant FeLV vaccine and a multivalent modified live virus vaccine with a chlamydial component. Vaccine. 2006;24:1838–46. DOIPubMedGoogle Scholar
- Sato Y, Mekata H, Sudaryatma PE, Kirino Y, Yamamoto S, Ando S, et al. Isolation of severe fever with thrombocytopenia syndrome virus from various tick species in area with human severe fever with thrombocytopenia syndrome cases. Vector Borne Zoonotic Dis. 2021;21:378–84. DOIPubMedGoogle Scholar
- Yoshikawa T, Shimojima M, Fukushi S, Tani H, Fukuma A, Taniguchi S, et al. Phylogenetic and geographic relationships of severe fever with thrombocytopenia syndrome virus in China, South Korea, and Japan. J Infect Dis. 2015;212:889–98. DOIPubMedGoogle Scholar
- Park E-S, Shimojima M, Nagata N, Ami Y, Yoshikawa T, Iwata-Yoshikawa N, et al. Severe Fever with Thrombocytopenia Syndrome Phlebovirus causes lethal viral hemorrhagic fever in cats. Sci Rep. 2019;9:11990. DOIPubMedGoogle Scholar
- Yu K-M, Jeong H-W, Park S-J, Kim Y-I, Yu M-A, Kwon H-I, et al. Shedding and transmission modes of severe fever with thrombocytopenia syndrome phlebovirus in a ferret model. Open Forum Infect Dis. 2019;6:
ofz309 . DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: February 14, 2023
Table of Contents – Volume 29, Number 3—March 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Tamaki Okabayashi, Center for Animal Disease Control, University of Miyazaki, 1-1 Gakuen-Kibanadai-Nishi, Miyazaki, 889-2192, Japan
Top