Volume 29, Number 3—March 2023
Synopsis
Yellow Fever Vaccine–Associated Viscerotropic Disease among Siblings, São Paulo State, Brazil
Figure 1
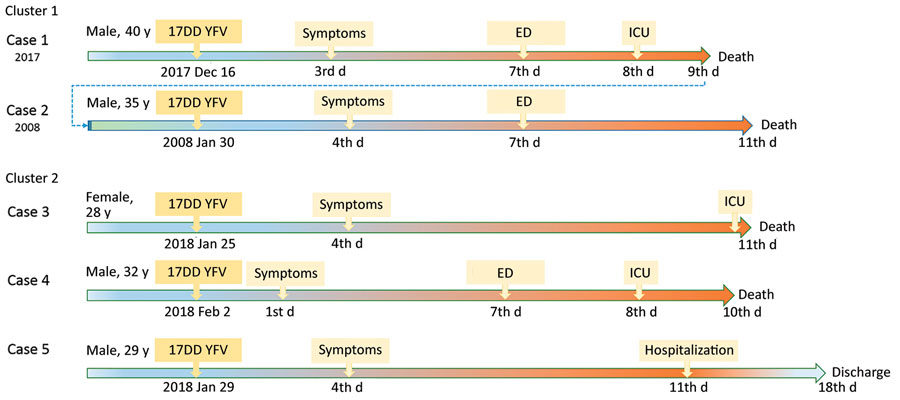
Figure 1. Timelines of reported cases of YFV–associated viscerotropic disease after 17DD vaccination during yellow fever epidemic, São Paulo state, Brazil, 2017–2018. Cases 1–2 were brothers and received standard doses. Cases 3–5 are siblings and received fractionated doses. ED, emergency department; ICU, intensive care unit; YFV, yellow fever vaccine.
Page created: January 04, 2023
Page updated: February 19, 2023
Page reviewed: February 19, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.