Volume 29, Number 3—March 2023
Dispatch
Emergomyces pasteurianus in Man Returning to the United States from Liberia and Review of the Literature
Abstract
A 65-year-old man with HIV sought treatment for fever, weight loss, and productive cough after returning to the United States from Liberia. Fungal cultures grew Emergomyces pasteurianus, and the patient’s health improved after beginning voriconazole. We describe the clinical case and review the literature, treatment, and susceptibilities for E. pasteurianus.
In March 2019, a 65-year-old man sought treatment at an emergency department in Virginia, USA, for fever, odynophagia, weight loss, and productive cough after returning from a 2-year stay in Liberia. In January 2019, he had been treated in Liberia for malaria, typhoid, and thrush. The patient already had an HIV diagnosis at the time he sought treatment, which we confirmed; he was taking lamivudine/zidovudine/nevirapine (150/300/200 mg/d) combination tablets, trimethoprim/sulfamethoxazole (160/800 mg/d) for pneumocystis prophylaxis, and fluconazole (100 mg/d) for thrush. Despite self-reported perfect compliance with his medication regimen, the patient lost 14 kg body weight and reported worsening fatigue over the 5-month period before he sought care in Virginia. The patient’s social history revealed smoking 30 packs/year and drinking up to 6 beers/day.
At initial workup, his CD4 T lymphocyte count was 16 cells/mm3 and HIV-1 viral RNA was 359 copies/mL. We excluded malaria during differential diagnosis with 3 thin/thick smears. Because the patient exhibited fever and was an immunocompromised returning traveler, we admitted him for further evaluation. Computed tomography (CT) of the chest revealed ground glass opacifications at bases, tree-in-bud nodularity within posterior, lateral, and anterior right upper lobes, and a central necrotic nodule at the left lower lung base measuring 1.3 × 2.1 cm (Appendix Figure). We found associated hilar and aortopulmonic lymphadenopathy measuring up to 8 mm in diameter. He was evaluated by infectious disease clinicians and started on amoxicillin/clavulanic acid (875/125 mg every 12 h) and doxycycline (100 mg every 12 h). We increased fluconazole to 200 mg/d and continued trimethoprim/sulfamethoxazole prophylaxis and antiretroviral therapy. The patient displayed night sweats and fever on days 2 (100.9°F) and 3 (101.5°F). He was afebrile on day 4 and for the remainder of his hospital stay.
A needle core biopsy of the lung nodule on day 4 revealed necrotizing granulomatous inflammation consisting of epithelioid histiocytes associated with intracellular narrow-based budding yeast (Figure 1, panels B, C) and multinucleated giant cells (Figure 1, panel A). Yeast forms 2–5 μm in size were visible on the hematoxylin and eosin smears. Both histochemical stains for Grocott methenamine silver and periodic acid–Schiff performed on the core biopsy were positive, but a mucicarmine stain was negative. Among the serologic fungal markers tested, serum cryptococcal antigen was negative. The Platelia Aspergillus galactomannan assay (Bio-Rad Laboratories, https://www.bio-rad.com) was elevated at 2.10 (reference range <0.49), and beta-D-glucan (Fungitell; Associates of Cape Cod, https://www.fungitell.com) was negative at 35 pg/mL (reference range <60 pg/mL). The patient was discharged on voriconazole (200 mg 2×/d) in addition to his HIV medication.
On day 17, the biopsy culture grew a fungus initially reported on the basis of morphology as presumptive Emmonsia sp., which was further identified through sequencing. We saw the patient for follow-up in the clinic on day 31; his appetite had returned, and he had gained 4 kg. We simplified his antiretroviral therapy to bictegravir/emtricitabine and tenofovir alafenamide. Day 37 culture results (LabCorp, https://www.labcorp.com) confirmed Emergomyces pasteurianus (formerly Emmonsia pasteuriana) through sequencing of internal transcribed spacer regions 1 and 2. MICs for antifungal agents were determined at the University of Texas Health Science Center (San Antonio, Texas, USA) by susceptibility tests at 23°C by broth microdilution (Table 1).
At day 81 follow-up, the patient reported that his cough had resolved. His CD4 was 54 cells/mm3 and viral load was 129 copies/mL. Our plan was to continue prescribing voriconazole for 12 weeks, then repeat the chest CT scan; however, the patient did not return for follow-up.
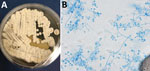
The geographic distribution of E. pasteurianus is still being described. Emergomyces is a dimorphic fungus related to Emmonsia, Histoplasma, and Blastomyces (1). This organism is an emerging pathogen among immunocompromised persons, especially those with HIV. E. pasteurianus was originally classified within the genus Emmonsia. However, the formation of yeast rather than adiaconidia (formerly adiaspores) and the clinical manifestations of emergomycosis suggested that E. pasteurianus belongs in a different genus from Emmonsia spp. (1). Subsequent genetic sequencing supported this relationship (2). There is evidence that the number of diagnosed emergomycosis cases are increasing, possibly because of more sensitive diagnostic techniques (1). We definitively diagnosed the infection in this patient through fungal cultures developed from lung biopsy samples, in which the organism readily grew as a filamentous fungi on Sabouraud dextrose agar, Mycocel agar, and brain–heart infusion agar at 25˚C. Colonies on Sabouraud dextrose agar incubated at 25˚C appeared white and compact and became domed/heaped over time (Figure 2, panel A). The reverse side started as white/cream and progressed to tan.
The microscopic appearance of the mold form of E. pasteurianus has been described as septate, hyaline hyphae, with short conidiophores arising at right angles that may show a slight swelling at the tip and typically produce >1 round conidia on short thin denticles. Conidia may also appear directly off the hyphae. The conidia are described as hyaline, thin-walled, 2–4 µm in size (2,3), which mirrors our experience. The yeast form (grown at 37˚C or present in tissue) is described as 2–5 µm with narrow budding. Bipolar budding and formation of giant cells with broad-based budding have also been reported (3–5); however, we did not definitively observe those forms in this case (Figure 2, panel B).
Clinical manifestations of emergomycosis may include dyspnea, pleuritic chest pain, and pink to purple nodular skin lesions (4). One study reported rapid progression of respiratory failure and death (5). Skin rash has been reported in some cases, but frequency of this clinical sign is unknown. CT imaging may show necrotizing cavitary lesions (4) or diffuse pulmonary infiltrates (5). Histopathology of skin lesions have shown yeast forms (4). Risk factors for emergomycosis include HIV (CD4 count <10 cells/mm3) (4), B-cell chronic lymphocytic leukemia with neutropenia, and chronic prednisone therapy (5). Chronic kidney disease was present in 2 case-patients, but association was uncertain (5).
Data are limited on antifungal susceptibilities for E. pasteurianus. The organism appears to have low MICs for itraconazole, posaconazole, and voriconazole but higher MICs for fluconazole and flucytosine (4). Although echinocandins generally have low MICs for the mold form of Emergomyces, activity in the pathogenic yeast form is less well known and significant discrepancies have been noted in other dimorphic fungi (6). A study of 50 clinical isolates of E. africanus demonstrated consistently low MICs to voriconazole, posaconazole, and itraconazole for both yeast and mold forms, with consistently elevated MICs for echinocandins and fluconazole (7). Although no guidelines exist to direct treatment for emergomycosis, multiple treatment courses have been used with varying outcomes (Table 2).
Serologic markers have been shown insufficient for diagnosing E. pasteurianus infection. The galactomannan assay was positive in the only previous case reporting a result and in our case (5). Beta-d-glucan testing was negative in our patient but was reported positive in 1/4 cases in other studies (8). Cross-reactivity with the histoplasma urine antigen has been reported (8). However, none of those tests are specific for Emergomyces, and they have not been systematically studied as markers for this specific pathogen.
Our study provides evidence of possible E. pasteurianus endemicity in Liberia and adds to the literature on susceptibilities for this emerging pathogen. We provide further evidence of low MICs to newer generation triazoles, suggesting their utility in empiric therapy, but additional data is needed to clarify formal breakpoints. Given the gaps in our knowledge about E. pasteurianus, public health providers should be aware of clinical manifestations of emergomycosis and consider it in the differential diagnosis, especially in regions where its presence is known.
Dr. Pierce was an infectious diseases fellow at Virginia Commonwealth University at the time of this work. He is currently a clinical assistant professor of medicine and serving as medical director of Infection Prevention for the Brody School of Medicine at East Carolina University, Greenville, North Carolina, USA. His research interests include mechanisms to decrease hospital-acquired infections and antimicrobial stewardship.
References
- Schwartz IS, Kenyon C, Feng P, Govender NP, Dukik K, Sigler L, et al. 50 years of Emmonsia disease in humans: the dramatic emergence of a cluster of novel fungal pathogens. PLoS Pathog. 2015;11:
e1005198 . DOIPubMedGoogle Scholar - Dukik K, Muñoz JF, Jiang Y, Feng P, Sigler L, et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). 2018;60:296–309.
- Samaddar A, Sharma A. Emergomycosis, an emerging systemic mycosis in immunocompromised patients: current trends and future prospects. Front Med (Lausanne). 2021;8:
670731 . DOIPubMedGoogle Scholar - Malik R, Capoor MR, Vanidassane I, Gogna A, Singh A, Sen B, et al. Disseminated Emmonsia pasteuriana infection in India: a case report and a review. Mycoses. 2016;59:127–32. DOIPubMedGoogle Scholar
- Gast KB, van der Hoeven A, de Boer MGJ, van Esser JWJ, Kuijper EJ, Verweij JJ, et al. Two cases of Emergomyces pasteurianus infection in immunocompromised patients in the Netherlands. Med Mycol Case Rep. 2019;24:5–8. DOIPubMedGoogle Scholar
- Dukik K, Al-Hatmi AMS, Curfs-Breuker I, Faro D, de Hoog S, Meis JF. Antifungal susceptibility of emerging dimorphic pathogens in the family Ajellomycetaceae. Antimicrob Agents Chemother. 2017;62:e01886–17.PubMedGoogle Scholar
- Maphanga TG, Britz E, Zulu TG, Mpembe RS, Naicker SD, Schwartz IS, et al. In vitro antifungal susceptibility of yeast and mold phases of isolates of dimorphic fungal pathogen Emergomyces africanus (formerly Emmonsia sp.) from HIV-infected South African patients. J Clin Microbiol. 2017;55:1812–20. DOIPubMedGoogle Scholar
- Gori S, Drouhet E, Guého E, Huerre MR, Lofaro A, Parenti M, et al. Cutaneous disseminated mycosis in a patient with AIDS due to a new dimorphic fungus [in French]. J Mycol Med. 1998;8:57–63.
- Pelegrín I, Ayats J, Xiol X, Cuenca-Estrella M, Jucglà A, Boluda S, et al. Disseminated adiaspiromycosis: case report of a liver transplant patient with human immunodeficiency infection, and literature review. Transpl Infect Dis. 2011;13:507–14. DOIPubMedGoogle Scholar
- Feng P, Yin S, Zhu G, Li M, Wu B, Xie Y, et al. Disseminated infection caused by Emmonsia pasteuriana in a renal transplant recipient. J Dermatol. 2015;42:1179–82. DOIPubMedGoogle Scholar
- Tang XH, Zhou H, Zhang XQ, Han JD, Gao Q. Cutaneous disseminated emmonsiosis due to Emmonsia pasteuriana in a patient with cytomegalovirus enteritis. JAMA Dermatol. 2015;151:1263–4. DOIPubMedGoogle Scholar
- Rooms I, Mugisha P, Gambichler T, Hadaschik E, Esser S, Rath PM, et al. Disseminated emergomycosis in a person with HIV infection, Uganda. Emerg Infect Dis. 2019;25:1750–1. DOIPubMedGoogle Scholar
- Capoor MR, Mishra N, Kolte S, Singla G, Gogna A, Rudramurthy S, et al. Disseminated Emergomyces pasteurianus infection in India: a case report and a review. Mycopathologia. 2020;185:193–200.PubMedGoogle Scholar
- Chik KK, To WK. Autochthonous Emergomyces pasteurianus pneumonia in an immunocompromised patient in Hong Kong: a case report. Hong Kong Med J. 2020;26:446–8. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: February 16, 2023
Table of Contents – Volume 29, Number 3—March 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jacob Pierce, East Carolina University, 2390 Hemby Lane, Greenville, NC 27834, USA
Top