Volume 29, Number 4—April 2023
Research
Effectiveness of BNT162b2 Vaccine against Omicron Variant Infection among Children 5–11 Years of Age, Israel
Figure 3
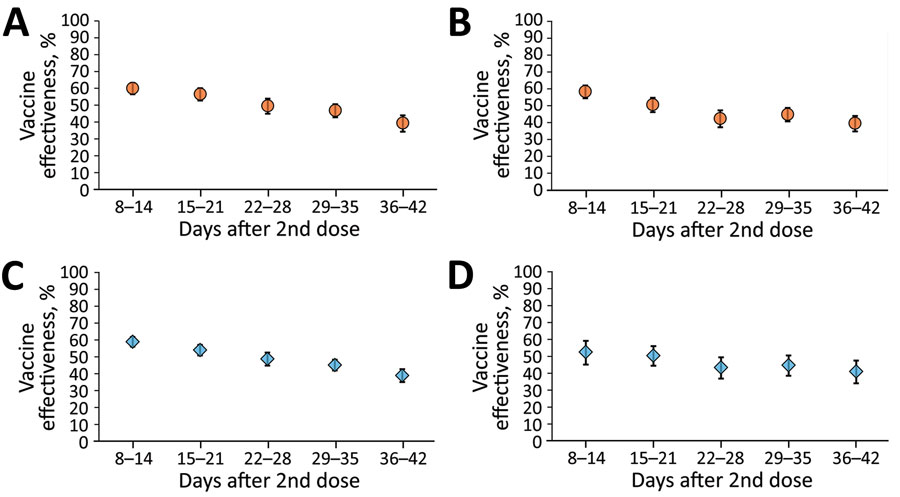
Figure 3. Sensitivity analyses for BNT162b2 (Pfizer BioNTech, https://www.biontech.com) vaccine effectiveness among children, Israel. A) Children 5–8 years of age, January 20–February 15, 2022; B) children 9–11 years of age, January 20–February 15, 2022; C) children 5–11 years of age, period 1 (January 20–February 2, 2022); D) children 5–11 years of age, period 2 (February 3–15, 2022). The center of each symbol is the point estimate; error bars indicate 95% CIs.
Page created: February 08, 2023
Page updated: March 20, 2023
Page reviewed: March 20, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.