Volume 29, Number 4—April 2023
Research Letter
Mpox in Young Woman with No Epidemiologic Risk Factors, Massachusetts, USA
Figure
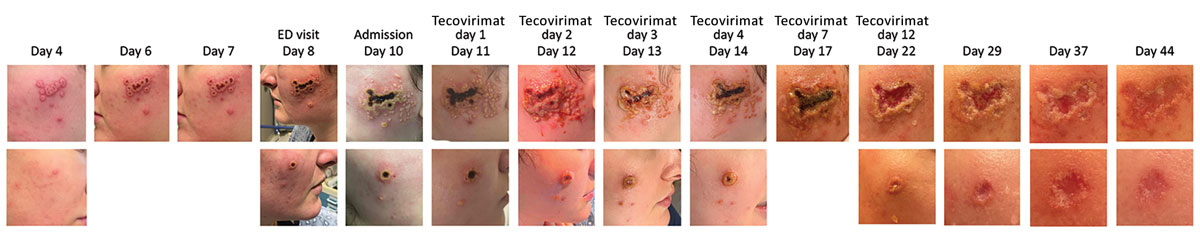
Figure. Progression of facial rash during mpox in a young woman in the absence of epidemiologic risk factors, Massachusetts, USA. Days since rash onset or beginning tecovirimat therapy are indicated. The rash began with pruritic erythematous macules on the bilateral infraorbital and malar areas, lower cutaneous lip, and chin and, by day 4, had progressed to vesicles followed by pustules on day 6 (top row, left cheek; bottom row, right cheek). On day 8 after rash onset, the patient had multiple confluent ulcers; macerated rolled borders were observed on the left cheek, and a single, large, deep-seated ulcer that had raised borders and a central hemorrhagic crust was observed on the right cheek. Satellite blisters and papules were present at early stages of ulcer development. The patient was started on tecovirimat on day 11 after rash onset, after which her lesions continued to evolve and had eventual loss of central eschar but persistent exudative, macerated borders by day 12 of tecovirimat therapy (day 22 after rash onset). Smaller lesions were treated with mupirocin ointment and dressed with loose gauze coverings. Toward the end of her 14-day treatment course (day 22), the escharotic ulcers developed granulated tissue. Ulcers had abundant granulated tissue and no central eschar and had begun to reepithelialize ≈2 weeks after completion of therapy (day 37).