Volume 29, Number 7—July 2023
Dispatch
Evolutionary Formation and Distribution of Puumala Virus Genome Variants, Russia
Cite This Article
Citation for Media
Abstract
We analyzed Puumala virus (PUUV) sequences collected from bank voles from different regions of Russia. Phylogenetic analysis revealed PUUV reassortments in areas with the highest hemorrhagic fever with renal syndrome incidence, indicating reassortment might contribute to pathogenic properties of PUUV. Continued surveillance is needed to assess PUUV pathogenicity in Russia.
In Eurasia, hantaviruses cause hemorrhagic fever with renal syndrome (HFRS), which is the most common zoonosis in Russia (1). During 2000–2017, a total of 131,590 HFRS cases were reported in Russia (2); most (≈98%) HFRS cases are caused by Puumala virus (PUUV) (2).
PUUV virions are enveloped particles containing 3 negative-sense single-strand RNA segments (3). Those segments vary in length; the large (L) segment is ≈6,550 nt long, the medium (M) segment is ≈3,650 nt, and the small (S) segment is ≈1,828 nt (4).
PUUV comprises 8 lineages: Central European, Alpe-Adrian, Danish, South-Scandinavian, North-Scandinavian, Finnish (FIN), Russian (RUS), and Latvian (LAT) (4–6). Modern PUUV diversity arose from multiple migrations of the viral host, the bank vole (Clethrionomys glareolus), during the postglacial period (7,8); that species also comprises several lineages: Ural, Eastern, Spanish, Italian, Balkan, Western, Carpathian, Basque, and Calabrian (9). The LAT, FIN, and RUS PUUV lineages likely had a common ancestor and originated from Eastern refugia (6). Those sublineages are carried by C. glareolus from Western, Eastern, and Carpathian lineages (10,11).
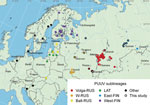
The RUS and FIN PUUV lineages are known to exist in Russia (4,8). Although PUUV-related HFRS has been reported from 54 regions of Russia (2), complete protein-coding sequences of the S segment in GenBank are only available from 8 regions: Kursk, Saratov, Samara, Tatarstan, Udmurtia, Bashkortostan, Omsk, and Karelia (Figure 1). Those sequences are mainly from viruses collected in the most epidemiologically active HFRS PUUV hotspots in the Volga region (2). PUUV genome variants in 46 other regions of Russia are still unknown. We investigated the distribution of different PUUV genome variants in Russia by obtaining PUUV sequences from bank voles captured in different regions.
We obtained lung tissue samples from bank voles collected by the territorial Сenters of Hygiene and Epidemiology in the Arkhangelsk, Ivanovo, Moscow, Penza, Saratov, Tyumen, Ulyanovsk, and Udmurtia regions (Figure 1). We stored samples, analyzed hantavirus antibodies by immunofluorescence assay (Table 1), extracted RNA, determined samples with high viral RNA content by real-time reverse transcription PCR (Table 1), and sequenced them as described in previous research (12).
To determine bank vole sublineages, we obtained sequences of the cytochrome b gene by using a previously described protocol (13). In all, we obtained PUUV genomic sequences and cytochrome b sequences from 17 bank voles (Appendix Table 1). All voles belonged to the Eastern lineage of C. glareolus (Appendix Figure 1).
We mapped locations of the newly obtained PUUV sequences and all representative LAT, FIN, and RUS lineage sequences available in GenBank that had complete S segment coding sequences (Figure 1). We also included representative sequences of other PUUV lineages on the map.
For phylogenetic analysis, we added sequences from this study to GenBank representatives of the LAT, FIN, and RUS lineages. We included representatives of the other PUUV lineages as outgroup (6) (Figure 2). According to S-segment phylogeny, we propose dividing the RUS lineage into 3 phylogenetically based sublineages: W-RUS (from western Russia) (12), Balt-RUS (from the Baltic coast region), and Volga-RUS (from the Volga River Valley). The percentages of identical nucleotides and amino acids within and between the sublineages did not enable us to establish clear criteria for the delimitation of the sublineages according to the quantitative differences (Table 2).
The topology of our S-segment tree does not match those from previous studies (12). The sequences we obtained provide additional information for better resolution of PUUV. L and S segments of PUUV are comparably conservative (Appendix Figure 2), and their phylogenetic trees mostly repeat each other. The M-segment tree was the most distinct (Figure 2, panel B), a finding that corresponds with previous data (5). That finding confirms that the M segment is the most variable and most often involved in reassortment events (14).
In the M-segment tree, the W-RUS sublineage stood within the Volga-RUS sublineage, but the branch from Bashkiria formed an outgroup (Figure 2, panel B). Almost the entire Volga-RUS sublineage, except for the Bashkir branch, appears to be a product of the exchange of the М segment between ancestors of the W-RUS and the Bashkir branch. The S and L segments are related to the Bashkir branch, and the M segment was obtained from the ancestors of the W-RUS sublineage. Those findings confirm and extend previous results (12). Thus, strains from the most epidemiologically active areas have arisen through reassortment. However, whether reassortment has altered the pathogenic properties of those strains remains unclear.
The genome variant from Penza belongs to the group that obtained the M segment by reassortment, as we have described (Figure 2, panel B). On the S-segment tree, Penza/Cg8730 is located on the Volga-RUS sublineage (Figure 2, panel A). However, on the L-segment tree, Penza/Cg8730 belongs to the W-RUS sublineage (Figure 2, panel C). Thus, Penza/Cg8730 likely emerged from a 2-stage reassortment. Although L segment exchange is not a unique event, it occurs much less frequently than exchange among other segments (14). Geographically, the Penza region lies west of locations where viruses belonging to the Volga-RUS sublineage were isolated but far from representatives of the W-RUS sublineage. Thus, Penza could be a meeting point of those 2 lineages.
On the S-segment tree, the FIN branch was represented by 2 sublineages: West-FIN from Finland and Karelia and East-FIN from western Siberia (Figure 2). However, on the M-segment tree, West-FIN and East-FIN did not form a common branch (Figure 2, panel B). The current hypothesis generally accepts that the FIN branch split into 2 parts when the territories were recolonized by bank voles after the glacier melted (8).
The sequences we obtained from the Arkhangelsk region clustered with sequences of viruses collected in Omsk and Tyumen regions, despite their geographic distance (Figure 1; Appendix Figure 3). According to the phylogenetic trees, the Tyumen and Omsk clades divided after the Arkhangelsk clade split from their common ancestor (Figure 2). Such branching suggests 2 possible paths for spread of the East-FIN subclade: from northwest Russia to the southeast, across the Ural Mountains to western Siberia; or spreading from the Pre-Ural region in 2 directions, to western Siberia and to the Arkhangelsk region. The second possibility supposes a second vole migration wave to the north, after PUUV split off from the East-FIN and West-FIN groups during postglacial period (8). PUUV sequences from the area between Arkhangelsk and western Siberia might shed more light on spread of the FIN lineage.
The newly identified viruses from the Tyumen and Arkhangelsk regions of Russia and viruses from Omsk form a common East-FIN branch. This finding raises additional questions about the dispersal routes of the FIN lineage.
Here we considered the RUS lineage as 3 separate sublineages: Volga-RUS, W-RUS, and Balt-RUS. The PUUV genome variants from Tatarstan, Udmurtia, Samara, and Saratov probably emerged through reassortment because they contained S and L segments related to the Bashkir branch and an M segment derived from ancestors of the W-RUS sublineage. The Penza/Cg8730 genome variant might have arisen from a 2-stage reassortment. Continued surveillance is needed to assess PUUV pathogenicity in Russia, but we found PUUV reassortments in areas with the highest HRFS incidence, indicating reassortment might contribute to pathogenic properties of PUUV.
Dr. Blinova is a research associate at Central Research Institute of Epidemiology, Moscow, Russia, and a PhD student in Russian Academy of Sciences, Moscow. Her research interests include epidemiology of Puumala and Dobrava/Belgrade viruses, HFRS incidence, and the course of diseases, phylogenetic diversity, and evolution of viruses.
Acknowledgments
We express our gratitude to the territorial Сenters of Hygiene and Epidemiology in the Arkhangelsk, Ivanovo, Moscow, Penza, Saratov, Tyumen, and Ulyanovsk regions, and Udmurtia republic for providing rodent material. We also express our gratitude to Anna Valdokhina and Victoria Bulanenko for sequencing implementation.
Immune diagnostics were funded by the Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products of Russian Academy of Sciences internal funds. Reverse transcription PCR, sequencing, and phylogenetic analysis were funded by the Federal Budget Institution of Science Central Research Institute of Epidemiology of The Federal Service on Customers Rights Protection and Human Wellbeing Surveillance internal funds. The other bioinformatic analysis, including mapping virus isolation points on interactive map, isolation by distance, and sliding window analyses, was funded by the Russian Science Foundation (grant no. 22-15-00230).
References
- Dzagurova TK, Siniugina AA, Ishmukhametov AA, Egorova MS, Kurashova SS, Balovneva MV, et al. Pre-clinical studies of inactivated polyvalent HFRS vaccine. Front Cell Infect Microbiol. 2020;10:
545372 . DOIPubMedGoogle Scholar - Tkachenko EA, Ishmukhametov AA, Dzagurova TK, Bernshtein AD, Morozov VG, Siniugina AA, et al. Hemorrhagic fever with renal syndrome, Russia. Emerg Infect Dis. 2019;25:2325–8. DOIPubMedGoogle Scholar
- Petazzi RA, Koikkarah AA, Tischler ND, Chiantia S. Detection of envelope glycoprotein assembly from old-world hantaviruses in the golgi apparatus of living cells. J Virol. 2021;95:e01238–20. DOIPubMedGoogle Scholar
- Kabwe E, Davidyuk Y, Shamsutdinov A, Garanina E, Martynova E, Kitaeva K, et al. Orthohantaviruses, emerging zoonotic pathogens. Pathogens. 2020;9:775. DOIPubMedGoogle Scholar
- Razzauti M, Plyusnina A, Niemimaa J, Henttonen H, Plyusnin A. Co-circulation of two Puumala hantavirus lineages in Latvia: a Russian lineage described previously and a novel Latvian lineage. J Med Virol. 2012;84:314–8. DOIPubMedGoogle Scholar
- Castel G, Chevenet F, Razzauti M, Murri S, Marianneau P, Cosson JF, et al. Phylogeography of Puumala orthohantavirus in Europe. Viruses. 2019;11:679. DOIPubMedGoogle Scholar
- Saxenhofer M, Weber de Melo V, Ulrich RG, Heckel G. Revised time scales of RNA virus evolution based on spatial information. Proc R Soc B Biol Sci. 2017;284(1860):20170857.
- Sironen T, Vaheri A, Plyusnin A. Molecular evolution of Puumala hantavirus. J Virol. 2001;75:11803–10. DOIPubMedGoogle Scholar
- Drewes S, Ali HS, Saxenhofer M, Rosenfeld UM, Binder F, Cuypers F, et al. Host-associated absence of human Puumala virus infections in northern and eastern Germany. Emerg Infect Dis. 2017;23:83–6. DOIPubMedGoogle Scholar
- Jeske K, Schulz J, Tekemen D, Balčiauskas L, Balčiauskienė L, Hiltbrunner M, et al. Cocirculation of Leptospira spp. and multiple orthohantaviruses in rodents, Lithuania, Northern Europe. Transbound Emerg Dis. 2022;69:e3196–201. DOIPubMedGoogle Scholar
- Rosenfeld UM, Drewes S, Ali HS, Sadowska ET, Mikowska M, Heckel G, et al. A highly divergent Puumala virus lineage in southern Poland. Arch Virol. 2017;162:1177–85. DOIPubMedGoogle Scholar
- Blinova E, Deviatkin A, Kurashova S, Balovneva M, Volgina I, Valdokhina A, et al. A fatal case of haemorrhagic fever with renal syndrome in Kursk Region, Russia, caused by a novel Puumala virus clade. Infect Genet Evol. 2022;102:
105295 . DOIPubMedGoogle Scholar - Schlegel M, Ali HS, Stieger N, Groschup MH, Wolf R, Ulrich RG. Molecular identification of small mammal species using novel cytochrome B gene-derived degenerated primers. Biochem Genet. 2012;50:440–7. DOIPubMedGoogle Scholar
- Klempa B. Reassortment events in the evolution of hantaviruses. Virus Genes. 2018;54:638–46. DOIPubMedGoogle Scholar
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 06, 2023
Table of Contents – Volume 29, Number 7—July 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ekaterina Blinova, Federal Service on Customers Rights Protection and Human Wellbeing Surveillance, 3a Novogireevskaya St., Moscow 111123, Russia
Top