Volume 29, Number 7—July 2023
Research
Systematic Review of Hansen Disease Attributed to Mycobacterium lepromatosis
Cite This Article
Citation for Media
Abstract
In 2008, bacilli from 2 Hansen disease (leprosy) cases were identified as a new species, Mycobacterium lepromatosis. We conducted a systematic review of studies investigating M. lepromatosis as a cause of HD. Twenty-one case reports described 27 patients with PCR–confirmed M. lepromatosis infection (6 dual M. leprae/M. lepromatosis): 10 case-patients in the United States (7 originally from Mexico), 6 in Mexico, 3 in the Dominican Republic, 2 each in Singapore and Myanmar, and 1 each in Indonesia, Paraguay, Cuba, and Canada. Twelve specimen surveys reported 1,098 PCR–positive findings from 1,428 specimens, including M. lepromatosis in 44.9% (133/296) from Mexico, 3.8% (5/133) in Colombia, 12.5% (10/80) in Brazil, and 0.9% (2/224) from the Asia-Pacific region. Biases toward investigating M. lepromatosis as an agent in cases of diffuse lepromatous leprosy or from Mesoamerica precluded conclusions about clinicopathologic manifestations and geographic distribution. Current multidrug treatments seem effective for this infection.
Since the pioneering work of Gerhard Armauer Hansen in the late 19th Century, Hansen disease (HD; also known as leprosy) has been attributed to Mycobacterium leprae. In 2008, bacilli from 2 cases of HD manifesting as diffuse lepromatous leprosy (DLL; also known as Lucio’s leprosy or diffuse leprosy of Lucio and Latapi), with signs of Lucio’s phenomenon (LP, erythema necroticans), were identified as a second causal agent of HD, M. lepromatosis (1). The M. lepromatosis genome has been sequenced and its evolution and genomic features in relation to M. leprae described elsewhere (2–5). Analyses indicated a most recent common ancestor ≈13.9 million years ago and a 9% overall difference in nucleotide sequence identity (7% in protein-coding genes, 18% in pseudogenes), differentiating M. lepromatosis as a separate species from M. leprae (3,4). Functional similarities, such as conservation of genes encoding for laminin binding and phenolic glycolipid 1 adhesin systems involved in Schwann cell invasion, outweigh differences, such as the presence of the hemN gene only in M. lepromatosis (3,4). Whether the 2 species differ systematically in clinicopathologic manifestations in humans has not yet been established, but validated real-time quantitative PCR assays based on unique repetitive elements in M. lepromatosis and M. leprae are now available (6).
DLL is a severe form of HD at the lepromatous pole of the spectrum characterized by an ineffective cellular immune response and high multibacillary load (7). Patients with DLL manifest diffuse nonnodular lesions and can develop LP, a severe reactional state in which recurrent crops of large and sharply demarcated ischemic or necrotic skin develop; the lesions often becoming ulcerated or even generalized, particularly on the legs, leading to secondary infection and, in some cases, fatal sepsis (8). DLL represents a higher proportion of HD cases in Mexico and the Caribbean than elsewhere, and studies reporting M. lepromatosis have tended to describe patients who originate from the region with that form of HD (9). However, dual M. leprae/M. lepromatosis and M. lepromatosis–only infections have also been reported beyond the Americas, principally in Asia.
Worldwide occurrences and clinical characteristics of HD attributed to M. lepromatosis infection since the species was identified have not been systematically reviewed. There is a clinical and scientific imperative to clarify the contribution of M. lepromatosis to a disease that greatly affects patient and public health. We report the results of a systematic review of reported HD cases with PCR–confirmed M. lepromatosis infection and data from surveys of archived PCR–tested specimens from persons affected by HD.
Review Protocol and Searches
The protocol for this systematic review was defined in advance and registered with PROSPERO, an international prospective register of systematic reviews (CRD42021239268). Database searches were performed on October 4, 2022 (Appendix). We imposed no date, language, or publication type restrictions. We manually searched bibliographies of all included studies.
Screening, Inclusion/Exclusion, and Quality Assessment
We conducted initial screening by title and abstract. We included references if a primary research study or clinical case report reported human infection with M. lepromatosis investigated using laboratory testing of current or archived specimens, irrespective of whether those specimens were positive for M. lepromatosis. We excluded animal studies and studies from before 2008, predating identification of M. lepromatosis. We excluded reviews and opinion pieces after manually checking bibliographies. Pairs of reviewers in parallel performed qualitative assessments to rate the methodologic quality of each included study as good, fair, or poor (Appendix). Reviewers used the Joanna Briggs Institute Critical Appraisal Tool for Case Reports (https://jbi.global/critical-appraisal-tools) and, for specimen surveys, a 9-item quality assessment tool adapted from the National Institutes of Health’s Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) (10).
Data Extraction and Analysis
Pairs of reviewers in parallel transferred extracted data into predefined templates. Data extracted from case reports were date and geographic location of testing, patient demographics, medical history, diagnostic methods and findings, treatment, and outcome. Data extracted from surveys were case information, test methods, source and type of specimens, and how many specimens provided DNA and tested positive for M. lepromatosis, M. leprae, or both.
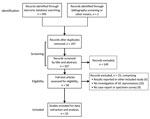
Database searches identified 495 references (Figure 1; Appendix). We identified no additional studies through bibliographic screening, but for completeness, we did include 2 case reports published after our database searches (9,11). After de-duplication and screening by title and abstract, we retained 58 studies, all published in peer-reviewed journals, for full text review; we extracted data from 33 (21 case reports, 12 specimen surveys). We excluded 1 because it was a retrospective review of 9 cases (12) that reported only 1 M. lepromatosis case that was also described in another source (13). Similarly, we excluded a review of cases among refugees and migrants in Italy during 2009–2018 (14) that reported PCR testing of 24 cases, 16 positive for M. leprae and 1 for M. lepromatosis, because the M. lepromatosis case was described in more detail in a case report (15).
Among the 21 case report studies, 14 studies described just 1 case, 6 described 2, and 1 described 6, yielding 32 PCR–positive cases: 21 M. lepromatosis–only, 5 M. leprae–only, and 6 dual infections (Table 1; Appendix). Of patients with M. lepromatosis, 10/27 resided in the United States (7 originally from Mexico), 6 in Mexico, 3 in the Dominican Republic, 2 each in Singapore and Myanmar, and 1 each in Indonesia, Paraguay, Cuba, and Canada. One study from Mexico reported 4 family cases, but only 2 were PCR–confirmed to be M. lepromatosis (9). Twenty-two cases occurred in the Americas and 5 in Asia; Mexico was the country of origin or residence for 13/27 case-patients. One source mentioned 2 case-patients from Costa Rica living in the United States but provided no details (23). Median age of case-patients was 41 years (range 21–86 years) and 63.0% (17/27) were male.
We assessed 13/21 studies as good and 8/21 as fair quality (Appendix). Eight studies did not provide detailed PCR methods (11,15,22,24–26,28,29), but 7 of these referred to laboratories (National Hansen’s Disease Programme; US Centers for Disease Control and Prevention; Ecole Polytechnique Fédérale de Lausanne; Japan Leprosy Research Centre) or involved authors with documented experience in M. lepromatosis diagnostic methods (11,22,24–26,28,29).
The case-patient from the study in which M. lepromatosis was first identified (1) was a patient originally from Mexico residing in the United States who had died from DLL with LP. PCR sequencing of the ≈1,500 bp 16S rRNA gene in acid-fast bacilli from frozen liver autopsy specimens showed that the strain, designated FJ924, matched most closely with M. leprae (BLAST analysis [https://blast.ncbi.nlm.nih.gov/Blast.cgi] of 16S rRNA gene, 1,475/1,506 bp, 97.9% identity) and next most closely with M. haemophilum (1,465/1,505 bp, 97.3%). The researchers obtained archived biopsy specimens from a second patient originally from Mexico, also with DLL and LP, who had died 5 years earlier. Gene sequences from that earlier case, including from the 16S rRNA gene, matched 100% with strain FJ924. On the basis of those findings, the researchers proposed a new species, M. lepromatosis, as a second causal agent of DLL, while speculating that it might also cause lepromatous (LL) and borderline lepromatous (BL) forms of HD (1). The researchers also obtained archived specimens from 2 fatal cases of DLL in Singapore (both case-patients died in 1999) with dual M. lepromatosis/M. leprae infection identified using a mix of species-specific and nonspecific primers matched to GenBank sequences (17).
In another study from Mexico, a sample, Mx1-22, taken from an 86-year-old patient with DLL and LP had rrs, rpoB, sigA, and hsp65 gene sequences identical to FJ924 (16). Subsequent studies used a range of species-specific primers and sequencing, 7 targeting 16S rRNA (13,18–21,23,30), 2 hemN (27,31), and 1 the LPMREP repetitive element (9) to confirm M. lepromatosis infection (Table 1). The oldest archived specimen in which M. lepromatosis was identified was from a US-resident patient originally from Mexico, 50 years of age, treated in Carville, Louisiana, USA, who was diagnosed with DLL with LP in 1963 (19). That patient, who initially sought treatment for a soft tissue sarcoma in the right lower leg, developed overt signs of DLL and LP after radiotherapy and amputation of the leg. Histopathologic review identified chronic HD lesions in the skin, vessels, and nerves surrounding the sarcoma, consistent with DLL. The patient survived to 85 years of age.
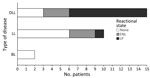
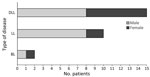
Of the 27 M. lepromatosis case-patients, 15 (55.6%) had DLL, 9 of whom also had LP; 10 had LL, 1 had LP, and 2 had BL (Figure 2). Among those cases, type 2 erythema nodosum leprosum (ENL) HD reactions were reported in 3 cases each of DLL and LL and in both BL cases. Male patients comprised 8/15 DLL and 1/2 BL case-patients but a higher proportion (8/10) of LL case-patients (Figure 3). Median time of evolution from initial symptoms to HD diagnosis was 2 years (range 8 months–12 years); 2 patients had been diagnosed with HD 20 and 28 years earlier. Two patients reported direct contact (hunting, handling, or eating) with armadillos in Mexico (23,25); a third came from a village in Mexico where armadillo meat was consumed, but the patient had not eaten it (20). Two patients in the United States had no known risk factors or exposures other than travel, including to Florida (18), worldwide travel including to Asia, the Caribbean, and the Middle East, and 2 trips to the Pacific coast of Mexico that were short but occurred consistent with a 7–8 year incubation period for HD manifestation (13).
Concomitant or differential diagnoses discussed in the case reports included sarcoidosis (initially treated with steroids) (19), syphilis (borderline positive antinuclear antibodies, treated initially with intramuscular penicillin) (23), rheumatoid arthritis (treated with prednisone and methotrexate 2 years earlier) (13), cutaneous vasculitis (treated with azathioprine and prednisone for >5 years) (30), vasculitis related to drug abuse (27), and acute kidney injury (11). All cases were otherwise consistent with the clinical and histopathologic picture of DLL (32): insidious onset with violaceous erythema developing on the face and lower extremities (may or may not be anesthetic); myxoedema-like aspect with smooth, tense, alopecic skin, progressing to madarosis; earlobe infiltration; rhinitis; nasal septal defects; hypohidrosis; xerotic and scaly skin with ichthyosiform appearance on lower limbs; areas of hypoesthesia and hyperesthesia associated with hypopigmented, atrophic plaques; and impaired sensation in the hands and feet becoming more generalized because of progressive nerve involvement. Histologically, dense histiocytic infiltration in skin and nerves was observed, advancing to endothelial proliferation with thickening of vascular walls, leading to occlusion of small arteries, and invasion of internal organs, indicated by hepatomegaly and splenomegaly.
All multibacillary HD case-patients were treated with multidrug therapy, typically with rifampicin, clofazimine, and dapsone (sometimes substituted with minocycline, clarithromycin, moxifloxacin, or oxfloxacin) for 12 or 24 months, plus corticosteroids (mainly prednisone), thalidomide, or both for ENL and LP. Treatment outcomes were favorable for 10/27 patients at time of reporting, although that group included 2 patients who had no regrowth of eyelashes or eyebrows at 3-year follow-up, 1 of whom also experienced ENL at 3 years (9). One patient whose mild neurologic deficits had resolved at 7 years was still taking thalidomide and prednisone because of new, although sparse, ENL lesions (23).
Eight patients were still receiving treatment or had just completed treatment at time of reporting. Most studies did not assess or report grade of disability. Six deaths were reported, of which 4 were attributed to DLL or sepsis secondary to DLL (1,17). One patient who died was a woman, 86 years of age, who improved after 10 days of treatment and was discharged after 2 weeks in stable condition but then died of unknown causes at home 3 months later (16). A man, 72 years of age, died from lung cancer after 5 months; he was described as having leprosy-like illness because, although 16S rRNA sequencing found a 100% match to M. lepromatosis and the patient manifested neurologic and dermatologic symptoms of LL and rhinorrhea, histopathology did not confirm mycobacteria within peripheral nerves (18).
Five case-patients tested positive only for M. leprae: One was a woman, 43 years of age, a United States resident originally from Nepal, diagnosed with midborderline HD with possible type 1 reaction and possible LP because of an erythematous geographic skin plaque which ulcerated, but was not biopsied (28). Another was a woman, 37 years of age, from Colombia, HIV-positive, diagnosed with LL and LP; the LP was potentially triggered as immune reconstitution inflammatory syndrome following initiation of antiretroviral therapy (22). The remaining 3 patients, from the Dominican Republic, 2 with LL and 1 with BL, were from a series of 6 cases that also included 1 M. lepromatosis–only and 2 dual-infection cases (31).
Quality assessment rated 7/12 specimen survey studies as good and 5/12 as fair quality. All but 1 study (42) reported details of PCR methods. Nine studies (34–42) used skin lesion or biopsy or tissue samples, 2 used both skin and lesion biopsy and skin slit smear specimens (6,33), and 1 did not explicitly state the sources of specimens (Table 2; Appendix) (3). Overall, surveys dated 1968–2020 reported 1,098 PCR–positive M. lepromatosis–only, M. leprae–only, or dual-infection findings from 1,428 specimens. M. lepromatosis was identified in 44.9% (133/296, 26 dual infection) of PCR–positive specimens from patients in Mexico or in the United States but originally from Mexico, 3.8% (5/133, 5 dual infection) of patients from Colombia, 12.5% (10/80, 3 dual infection) of patients from Brazil, and 0.9% (2/224) of patients from the Pacific-Asia region; all 157 specimens from China, 50 from Africa (Mali, Uganda), and 77 from Venezuela were positive only for M. leprae. For patients from Mexico, excluding those resident in the United States, M. lepromatosis was detected in 43.9% (116/264) of PCR–positive specimens, including 25 dual infections. For patients resident in the United States from any country of origin, M. lepromatosis was detected in 16.7% (20/120) of PCR–positive specimens, including 1 dual infection.
The distribution of HD types among 116 M. lepromatosis–only and 13 dual-infection patients was tuberculoid in 7 (5.4%); borderline tuberculoid, midborderline, or borderline lepromatous in 20 (15.5%); LL in 73 (56.6%); and DLL in 29 (22.5%). LP was reported in relation to 14/27 specimens from patients in Mexico or in the United States but originally from Mexico. One patient from Mexico with LL who provided a specimen positive for M. lepromatosis had consumed armadillo meat (40).
Our systematic review identified 27 case reports of HD attributed to PCR–confirmed M. lepromatosis infections. In addition, surveys of specimens from current patients and archived material uncovered 153 cases of M. lepromatosis HD. Most of those infections (60% of case reports, 87% of surveyed specimens) occurred in patients resident in Mexico or in the United States but originally from Mexico. Most (70%) of the case reports described patients with DLL, among whom half manifested LP.
Our findings appear to substantiate the hypothesis that M. lepromatosis is the predominant HD pathogen in Mesoamerica and the Caribbean, and particularly in Mexico, and that it has a strong tendency to cause the DLL form of HD and, indirectly, severe LP reaction. However, there are some important caveats. First, DLL and LP were identified in Mexico in the late 19th Century by physicians Lucio Nájera and Ygnacio Alvarado and were further characterized by Fernando Latapí in 1938 (43,44). Discovery of M. lepromatosis in fatal cases of DLL with LP in 2 patients in the United States who were originally from Mexico (1), combined with the high proportion of HD cases in Mexico that were DLL, with or without LP, might have resulted in disproportionate publication of case reports and specimen surveys focused on this form of HD in this region. Laboratory expertise and resources for detecting M. lepromatosis are also more readily available in Mexico and the United States. However, Mexico is not an HD-endemic country, reporting an average of <200 newly detected cases per year during 2005–2021 (45), mostly in the states of Guerrero, Jalisco, Oaxaca, Sinaloa, and Michoacán (46). Although this annual average represents a relatively small number of cases, in the context of HD elimination, it is a matter of public health concern. In addition, M. lepromatosis has a tendency to cause severe forms including DLL, which with its nonnodular manifestation is prone to diagnosis at later stages; therefore, there remains an immense personal impact on persons affected by the disease.
Current HD multidrug therapies appear to be effective treatments, except in the most severe cases in which patients are at risk of secondary infection. However, evidence on the apparent effectiveness of current multidrug therapy regimens in treating HD caused by M. lepromatosis is constrained by the small number of cases described, their clinical complexity and severity, and lack of follow-up data to characterize long-term outcomes, including permanent disabilities.
Our review showed that M. lepromatosis–caused HD occurs in other countries in the Americas and, sporadically, in Asia and the Pacific. Most notably, 1 in 8 specimens from the south of Brazil were identified as M. lepromatosis. Brazil is an HD-endemic country with ≈20,000 newly detected cases per year. Also, the survey data in our review showed that, when type of HD was reported, a higher percentage of cases attributed to M. lepromatosis were LL (57%) than DLL (23%). Even a small fraction of HD cases in Brazil caused by M. lepromatosis would represent a large number of cases. The clear implication is that a national survey of current and newly detected HD cases in Brazil is needed, ideally using the recently validated M. lepromatosis/M. leprae diagnostic assay (6). Parallel studies in neighboring countries where M. lepromatosis and DLL are perhaps more prevalent, would yield sequence data that could be used to investigate the distribution of M. lepromatosis variants and lineages, including drug-resistant strains, to achieve the same level of understanding as for M. leprae (5,47). Whether M. lepromatosis has a pathogenic tendency toward causing DLL and whether certain population groups are more susceptible to developing this form of HD can be investigated by pathogen and host genomic testing across the HD spectrum and in different populations. From a One Health perspective, we know that zoonotic transmission of M. leprae presents a risk to human health (48), and M. lepromatosis has been detected in red squirrels (Sciurus vulgaris) from the British Isles, including Ireland (49). Given that 4 case-patients with HD caused by M. lepromatosis in our review had direct or indirect contact with wild armadillos, a survey of archived specimens or specimens from freshly caught armadillos in Mexico and Brazil is warranted (50).
The narrowly focused scope, sensitivity, and specificity of lepromatosis as a search term and the relatively few references included in our review give us confidence that all relevant studies were identified. Quality of reporting was good in 61% of included studies and fair in the remainder. The tendency of studies to focus on DLL in Mesoamerica, possibly resulting in observational and publication biases for case reports and sampling bias for surveys, were the main sources of bias in our review, although there were several large studies from other regions with null findings for M. lepromatosis. A key quality item considered for this review was adequate description of PCR methods, which most, but not all, studies provided. Specimen surveys more consistently described PCR methods, including targets and primers, than did case reports, some of which covered time periods during which those methods were still being developed. M. lepromatosis does not manifest only as DLL, but most specimen surveys did not provide clinical data for the patients sampled. Even when HD type was stated, misclassification was possible unless HD specialists or reference centers were involved in diagnosis. Although we cannot entirely preclude the possibility of double counting, we identified only a few cases that were reported twice and contacted the authors of 2 studies when geography and time span suggested that possibility to confirm that there was no overlap (33,34).
It is perhaps remarkable that a new species causing an endemic disease of major public health impact has not prompted larger-scale studies to determine its true prevalence. Even if options for patient management are determined by clinical manifestations of HD rather than its etiologic agents, understanding disease attribution and distribution of a highly pathogenic species are clearly important, and the availability of validated PCR methods enables large-scale epidemiologic studies to be conducted.
In conclusion, clinicians need to be aware that Hansen disease of various forms can be caused by either M. leprae or M. lepromatosis. Current multidrug therapy regimens appear to be effective regardless of infecting species.
Dr. Collin is a public health epidemiologist in England, with expertise in epidemiology of infectious diseases, and a visiting professor at the Federal University of Espírito Santo, Brazil.
References
- Han XY, Seo YH, Sizer KC, Schoberle T, May GS, Spencer JS, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856–64. DOIPubMedGoogle Scholar
- Han XY, Sizer KC, Thompson EJ, Kabanja J, Li J, Hu P, et al. Comparative sequence analysis of Mycobacterium leprae and the new leprosy-causing Mycobacterium lepromatosis. J Bacteriol. 2009;191:6067–74. DOIPubMedGoogle Scholar
- Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, et al. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proc Natl Acad Sci U S A. 2015;112:4459–64. DOIPubMedGoogle Scholar
- Silva FJ, Santos-Garcia D, Zheng X, Zhang L, Han XY. Construction and analysis of the complete genome sequence of leprosy agent Mycobacterium lepromatosis. Microbiol Spectr. 2022;10:
e0169221 . DOIPubMedGoogle Scholar - Avanzi C, Singh P, Truman RW, Suffys PN. Molecular epidemiology of leprosy: An update. Infect Genet Evol. 2020;86:
104581 . DOIPubMedGoogle Scholar - Sharma R, Singh P, McCoy RC, Lenz SM, Donovan K, Ochoa MT, et al. Isolation of Mycobacterium lepromatosis and development of molecular diagnostic assays to distinguish Mycobacterium leprae and M. lepromatosis. Clin Infect Dis. 2020;71:e262–9. DOIPubMedGoogle Scholar
- Scollard DM. Pathogenesis and pathology of leprosy. In: Scollard DM, Gillis TP, editors. International textbook of leprosy. 2018. https://www.internationaltextbookofleprosy.org/chapter/pathology
- Frade MAC, Coltro PS, Filho FB, Horácio GS, Neto AA, da Silva VZ, et al. Lucio’s phenomenon: A systematic literature review of definition, clinical features, histopathogenesis and management. Indian J Dermatol Venereol Leprol. 2022;88:464–77. DOIPubMedGoogle Scholar
- Romero-Navarrete M, Arenas R, Han XY, Vega-Memije ME, Castillo-Solana AD. Leprosy caused by Mycobacterium lepromatosis. Am J Clin Pathol. 2022;158:678–86. DOIPubMedGoogle Scholar
- National Heart Lung and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies [cited 2019 May 24]. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Norman T, Zikry J, Worswick S, Kim G, Ochoa MT. Lucio phenomenon with concomitant necrotizing fasciitis and acute kidney injury. Dermatol Online J. 2022;28:28. DOIPubMedGoogle Scholar
- Bezalel SA, Onajin O, Gonzalez-Santiago TM, Patel R, Pritt BS, Virk A, et al. Leprosy in a midwestern dermatology clinic: report of 9 patients. Mayo Clin Proc. 2019;94:417–23. DOIPubMedGoogle Scholar
- Virk A, Pritt B, Patel R, Uhl JR, Bezalel SA, Gibson LE, et al. Mycobacterium lepromatosis lepromatous leprosy in US citizen who traveled to disease-endemic areas. Emerg Infect Dis. 2017;23:1864–6. DOIPubMedGoogle Scholar
- Beltrame A, Barabino G, Wei Y, Clapasson A, Orza P, Perandin F, et al. Leprosy in refugees and migrants in Italy and a literature review of cases reported in Europe between 2009 and 2018. Microorganisms. 2020;8:1113. DOIPubMedGoogle Scholar
- Trave I, Barabino G, Cavalchini A, Parodi A. Long-term ulcerations caused by Mycobacterium lepromatosis. Int J Mycobacteriol. 2020;9:223–5. DOIPubMedGoogle Scholar
- Vera-Cabrera L, Escalante-Fuentes WG, Gomez-Flores M, Ocampo-Candiani J, Busso P, Singh P, et al. Case of diffuse lepromatous leprosy associated with “Mycobacterium lepromatosis”. J Clin Microbiol. 2011;49:4366–8. DOIPubMedGoogle Scholar
- Han XY, Sizer KC, Tan HH. Identification of the leprosy agent Mycobacterium lepromatosis in Singapore. J Drugs Dermatol. 2012;11:168–72.PubMedGoogle Scholar
- Jessamine PG, Desjardins M, Gillis T, Scollard D, Jamieson F, Broukhanski G, et al. Leprosy-like illness in a patient with Mycobacterium lepromatosis from Ontario, Canada. J Drugs Dermatol. 2012;11:229–33.PubMedGoogle Scholar
- Han XY, Jessurun J. Severe leprosy reactions due to Mycobacterium lepromatosis. Am J Med Sci. 2013;345:65–9. DOIPubMedGoogle Scholar
- Han XY, Quintanilla M. Diffuse lepromatous leprosy due to Mycobacterium lepromatosis in Quintana Roo, Mexico. J Clin Microbiol. 2015;53:3695–8. DOIPubMedGoogle Scholar
- Widiatma RR, Sukanto H. Diffuse lepromatous leprosy caused by dual infection of Mycobacterium leprae and Mycobacterium lepromatosis: a case report. Dermatol Rep. 2019;11:180–2. DOIGoogle Scholar
- Serrano-Coll HA, Beltrán-Alzate JC, Buitrago SM, Cardona-Castro N. Lepromatous leprosy and human immunodeficiency virus co-infection associated with phenomenon of Lucio versus immune reconstitution inflammatory syndrome. Infectio. 2016;20:272–5. DOIGoogle Scholar
- Sotiriou MC, Stryjewska BM, Hill C. Two cases of leprosy in siblings caused by Mycobacterium lepromatosis and review of the literature. Am J Trop Med Hyg. 2016;95:522–7. DOIPubMedGoogle Scholar
- Velarde-Félix JS, Alvarado-Villa G, Vera-Cabrera L. “Lucio’s phenomenon” associated with Mycobacterium lepromatosis. Am J Trop Med Hyg. 2016;94:483–4.PubMedGoogle Scholar
- Cleary LC, Suraj S, Haburchak D, Turrentine JE. The armadillo factor: lepromatous leprosy. Am J Med. 2017;130:1163–6. DOIPubMedGoogle Scholar
- Htet L, Kai M, Miyamoto Y. New etiology of leprosy in Myanmar: another two patients. Lepr Rev. 2018;89:316–8. DOIGoogle Scholar
- Aldama Olmedo OM, Escobar M, Martínez MJ, Aldama M, Montoya Bueno C, Celias LF, et al. Necrotizing erythema nodosumin lepromatous leprosy associated with mixed infection by Mycobacterium lepromatosis and Mycobacterium leprae [in Spanish]. Rev Nac (Itauguá). 2020;12:107–15. DOIGoogle Scholar
- Oo YM, Paez A, Brown R. Leprosy: A rare case of infectious peripheral neuropathy in the United States. IDCases. 2020;20:
e00765 . DOIPubMedGoogle Scholar - Watson W, Vassantachart JM, Luke J. Clinicopathological challenge: acute blistering and dermal papules in a patient with scleroderma. Int J Dermatol. 2020;59:e99–101. DOIPubMedGoogle Scholar
- Flores-Suárez LF, Fernández-Sánchez M, Ahumada-Topete VH, Rodríguez M, Charli-Joseph Y. After all, still a magnificent impersonator. Rheumatology (Oxford). 2021;60:e245–6. DOIPubMedGoogle Scholar
- Fernández JDP, Pou-Soarez VE, Arenas R, Juárez-Duran ER, Luna-Rojas SL, Xicohtencatl-Cortes J, et al. Mycobacterium leprae and Mycobacterium lepromatosis infection: a report of six multibacillary cases of leprosy in the Dominican Republic. Jpn J Infect Dis. 2022;75:427–30. DOIPubMedGoogle Scholar
- Kumar DP, Uprety S, Dogra S. Clinical diagnosis of leprosy. In: Scollard DM, Gillis TP, editors. International textbook of leprosy. 2018 [cited 2023 Jan 8]. https://www.internationaltextbookofleprosy.org/chapter/diagnosis-leprosy
- Cardona-Castro N, Escobar-Builes MV, Serrano-Coll H, Adams LB, Lahiri R. Mycobacterium lepromatosis as cause of leprosy, Colombia. Emerg Infect Dis. 2022;28:1067–8. DOIPubMedGoogle Scholar
- Fragozo-Ramos MC, Cano-Pérez E, Sierra-Merlano RM, Camacho-Chaljub F, Gómez-Camargo D. Clinical, histopathological, and molecular characterization of leprosy in an endemic area of the colombian caribbean. Int J Mycobacteriol. 2021;10:155–61.PubMedGoogle Scholar
- Han XY, Sizer KC, Velarde-Félix JS, Frias-Castro LO, Vargas-Ocampo F. The leprosy agents Mycobacterium lepromatosis and Mycobacterium leprae in Mexico. Int J Dermatol. 2012;51:952–9. DOIPubMedGoogle Scholar
- Han XY, Aung FM, Choon SE, Werner B. Analysis of the leprosy agents Mycobacterium leprae and Mycobacterium lepromatosis in four countries. Am J Clin Pathol. 2014;142:524–32. DOIPubMedGoogle Scholar
- Kai M, Fafutis-Morris M, Miyamoto Y, Mukai T, Mayorga-Rodriguez J, Rodriguez-Castellanos MA, et al. Mutations in the drug resistance-determining region of Mycobacterium lepromatosis isolated from leprosy patients in Mexico. J Dermatol. 2016;43:1345–9. DOIPubMedGoogle Scholar
- Khan S, Adler BL, Armstrong AW, Lahiri R, Ochoa MT. Impact of Mycobacterium leprae and Mycobacterium lepromatosis on immune reactions and clinical outcomes in Hansen’s disease: A single-center retrospective analysis. J Am Acad Dermatol. 2023;88:722–4. DOIPubMedGoogle Scholar
- Torres-Guerrero E, Sánchez-Moreno EC, Atoche-Diéguez CE, Carrillo-Casas EM, Arenas R, Xicohtencatl-Cortes J, et al. Identification of Mycobacterium leprae and Mycobacterium lepromatosis in formalin-fixed and paraffin-embedded skin samples from Mexico. Ann Dermatol. 2018;30:562–5. DOIPubMedGoogle Scholar
- Vera-Cabrera L, Escalante-Fuentes W, Ocampo-Garza SS, Ocampo-Candiani J, Molina-Torres CA, Avanzi C, et al. Mycobacterium lepromatosis Infections in Nuevo León, Mexico. J Clin Microbiol. 2015;53:1945–6. DOIPubMedGoogle Scholar
- Yuan Y, Wen Y, You Y, Xing Y, Li H, Weng X, et al. Characterization of Mycobacterium leprae genotypes in China—identification of a new polymorphism C251T in the 16S rRNA gene. PLoS One. 2015;10:
e0133268 . DOIPubMedGoogle Scholar - Zhang Y, Sun Y, Wang C, Liu D, Chen M, Fu X, et al. Failure to detect Mycobacterium lepromatosis as a cause of leprosy in 85 Chinese patients. Indian J Dermatol Venereol Leprol. 2015;81:499–500. DOIPubMedGoogle Scholar
- Saúl A, Novales J. [Lucio-Latapí leprosy and the Lucio phenomenon]. Acta Leprol. 1983;1:115–32.PubMedGoogle Scholar
- Vargas-Ocampo F. Diffuse leprosy of Lucio and Latapí: a histologic study. Lepr Rev. 2007;78:248–60. DOIPubMedGoogle Scholar
- World Health Organization. The Global Health Observatory. Geneva: The Organization; 2022 [cited 2023 Jan 8]. https://www.who.int/data/gho
- Larrea MR, Carreño MC, Fine PE. Patterns and trends of leprosy in Mexico: 1989-2009. Lepr Rev. 2012;83:184–94. DOIPubMedGoogle Scholar
- Aubry A, Sammarco Rosa P, Chauffour A, Fletcher ML, Cambau E, Avanzi C. Drug resistance in leprosy: An update following 70years of chemotherapy. Infect Dis Now. 2022;52:243–51. DOIPubMedGoogle Scholar
- Deps P, Antunes JMAP, Collin SM. Zoonotic risk of Hansen’s disease from community contact with wild armadillos: A systematic review and meta-analysis. Zoonoses Public Health. 2021;68:153–64. DOIPubMedGoogle Scholar
- Avanzi C, Del-Pozo J, Benjak A, Stevenson K, Simpson VR, Busso P, et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science. 2016;354:744–7. DOIPubMedGoogle Scholar
- Deps P, Collin SM. Mycobacterium lepromatosis as a second agent of Hansen’s disease. Front Microbiol. 2021;12:
698588 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 09, 2023
Table of Contents – Volume 29, Number 7—July 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Patrícia Deps, Universidade Federal do Espírito Santo, Vitória, Espírito Santo, Brazil
Top