Volume 29, Number 7—July 2023
Synopsis
Multicentric Case Series and Literature Review of Coccidioidal Otomastoiditis
Abstract
Coccidioidomycosis involving the ear, mastoid bone, or both is uncommon. We describe 5 new cases from the United States and review 4 cases reported in the literature of otomycosis and mastoiditis caused by Coccidioides. Of the 9 cases, 8 were linked to residence in or travel to California. Two patients had poorly controlled diabetes mellitus, 7 had otomastoiditis, 1 had otitis externa without mastoid involvement, and 1 had mastoiditis without otic involvement. Four patients had concurrent or prior pulmonary coccidioidomycosis. Ipsilateral facial nerve palsies developed in 2 patients. All patients received antifungal treatment for varying durations, and 8 of the 9 patients underwent surgical debridement. Clinicians should consider coccidioidomycosis as a differential diagnosis for otomastoiditis in patients with geographic risks.
The clinical manifestations of coccidioidomycosis, an expanding endemic mycosis caused by Coccidiodes immitis and C. posadasii (1), are notoriously protean (2,3). Infection occurs primarily by inhalation of aerosolized arthroconidia, which undergo morphologic change within the lungs and turn into spherules (large structures containing endospores) (4). Coccidioidomycosis is a highly variable illness and may be asymptomatic or cause a mild respiratory illness in up to 60% of infected persons and an uncomplicated pulmonary infection in most others (4). Dissemination or progressive pulmonary disease affects 1%–2% of persons infected with Coccidioides spp. (2). In those persons, after spherule rupture, endospores may spread hematogenously or through the lymphatic system to virtually all organs, although extrapulmonary clinical disease at sites other than the brain, skin, bone, or psoas muscle is uncommon (5). Those infrequently encountered sites of disease may present diagnostic and therapeutic challenges (2).
Coccidioidomycosis involving the middle or outer ear, mastoid bone, or both is uncommon. We describe 5 cases of otomycosis and mastoiditis caused by Coccidioides spp. in the United States and review 4 cases reported in the literature. Institutional Review Board ethics approval was obtained at Kern Medical Center (Bakersfield, California, USA) and was not required for unidentified case reports at the other medical centers involved.
Case 1
In 2019, a 76-year-old White man from California sought care for left-sided hearing loss that started after an eschar developed on the left tragus and cheek. He had no history of trauma. An otolaryngologist noted a middle ear effusion, which was managed conservatively with topical therapies and tympanostomy tube insertion. Because the effusion persisted despite those interventions, the otolaryngologist sent samples for microbiological analysis, including bacterial and fungal cultures. Bacterial cultures were negative; however, after 3 days of incubation, a mold was isolated, identified by DNA Probe (Hologic, Inc., https://www.hologic.com) as Coccidioides spp. A chest radiograph was unremarkable. Oral fluconazole (400 mg/d) was started. Although the effusion decreased, hearing did not improve. After 3 months of therapy, fluconazole was discontinued because of a widespread maculopapular exanthem. Two months thereafter, the unilateral hearing loss persisted. At that time, computed tomography (CT) of the head showed complete opacification of the left mastoid air cells, a soft tissue infiltrate within the middle ear chambers involving the epitympanic recess, and thinning of the left mastoid bone without obvious bony destruction or intracranial extension. Otoscopic examination revealed extensive debris and discharge throughout the ear canal and tympanic membrane perforation. The ear canal was debrided biweekly for 3 months, which led to healing of the membrane and decreased erythema and discharge from the ear canal. Subsequent treatment was itraconazole (200 mg by mouth 2×/d for 6 months). The patient’s hearing partially improved after several weeks of antifungal treatment; improvement reached a plateau at 5 months but did not return to baseline. At the end of therapy, the patient was no longer available for follow-up.
Case 2
In 2015, a 52-year-old man from California sought care for headache and jaw pain. Findings from a physical examination, including the oropharynx and tympanic membranes, were unremarkable. Initially, nonsteroidal antiinflammatories were prescribed, and the condition was managed expectantly. Over the next 4 months, the intensity of the patient’s symptoms increased and the pain localized to the left mastoid. Magnetic resonance imaging (MRI) demonstrated left mastoiditis. There was no clinical or radiographic evidence of pulmonary involvement. A biopsy sample was collected from the mastoid; bacterial and fungal cultures were negative, but Coccidioides spherules were identified by histopathologic examination. Subsequently, serologic testing demonstrated a Coccidioides complement fixation (CF) titer of 1:8, and Coccidioides immunodiffusion was positive for IgG. Treatment with oral fluconazole (400 mg/d) was initiated. At a follow-up visit 2 years later, the patient had residual mastoid pain but no other signs or symptoms of disease, and his CF titer had decreased to 1:2. Repeated imaging after 26 months revealed substantial improvement, but radiographic evidence of mastoiditis persisted. Fluconazole was continued indefinitely, although the patient has since been unavailable for follow-up.
Case 3
In 1999, a 42-year-old White man from California sought care for right ear fullness and tinnitus. Examination indicated a right middle ear effusion without erythema. Systemic antihistamines were prescribed; however, the patient returned 1 month later without clinical improvement. Sequential empiric courses of oral prednisone (20 mg/d for 14 days) and amoxicillin/clavulanate (500/125 mg 3×/d for 10 days) also did not lead to improvement. Soon thereafter, ipsilateral facial nerve palsy developed. MRI revealed evidence of an ongoing middle ear effusion and mastoiditis. Tympanocentesis was performed, and C. immitis was identified on cultures of the aspirated middle-ear fluid. Serum Coccidioides CF titer was 1:4, and Coccidioides immunodiffusion was positive for IgG. After further questioning, the patient recalled having had an episode of subacute pneumonia ≈18 months earlier that did not respond to levofloxacin. After Coccidioides otomastoiditis was diagnosed, oral fluconazole (800 mg/d) was prescribed. Treatment was continued for 3 years, the ear effusion and tinnitus resolved, the 7th nerve palsy partially improved, the CF titer declined to undetectable, and the radiographic appearance of the mastoiditis improved. After 3 years, the patient self-discontinued fluconazole. He has remained asymptomatic with undetectable CF titers through 21 years of follow-up.
Case 4
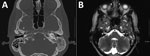
In 2014, a 22-year-old Hispanic man from Bakersfield, California, in the Cocciodioides epicenter of San Joaquin Valley, who had a medical history of uncontrolled diabetes mellitus type 1, received a diagnosis of pulmonary and osteoarticular coccidioidomycosis but was nonadherent to therapy with fluconazole and then posaconazole (substituted because of liver injury). Peak CF titer was 1:256. After 1 year of nonadherence, he sought care for left ear pain and purulent drainage for 5 days. Symptom onset was gradual, constant, aching, and sharp, with radiation to left jaw and left eye. Left otitis externa was diagnosed and treated with oral amoxicillin and topical neomycin, polymyxin B sulfates, and hydrocortisone otic solutions. He was unavailable for follow-up for another 6 months, at which point he sought care for progressive left-sided hearing loss of 1 week’s duration and diffuse pounding headache, nausea, and vomiting for 1 month. Neurologic examination suggested left conductive hearing loss. Results for other cranial nerves were unremarkable. Otoscopic examination revealed that the left tympanic membrane was erythematous and bulging with a middle ear effusion. Results of lumbar puncture were unremarkable. Coccidioides CF at this time was 1:64. CT showed complete opacification of the mastoid air cell system on the left side with fluid in the middle ear (Figure 1, panel A). MRI showed left mastoiditis with an extradural collection of fluid. The patient underwent a left myringotomy with insertion of a tympanostomy tube. Ten days postoperatively, he remained symptomatic, reporting a constant headache radiating from his left eye to the occipital area, along with light-headedness and left ear hearing loss. Repeat CT imaging 2 weeks after the myringotomy suggested persistent left otomastoiditis. MRI showed heterogenous signal in the left mastoid and middle ear and discontinuity of the roof of the left mastoid but no temporal lobe or middle cranial fossa involvement (Figure 1, panel B). Because of the lack of response to tympanostomy tube placement, a left mastoidectomy and tympanoplasty were performed. Mastoid tissue was cultured, and C. immitis grew after 28 days. The patient was hospitalized, and a 6-week course of intravenous liposomal amphotericin B monotherapy was initiated, which he continued as an outpatient. At 6-week follow-up, his hearing had gradually increased. Antifungals were changed to posaconazole (400 mg/d orally). One year later, Coccidioides CF had improved to 1:8, and the patient remained well.
Case 5
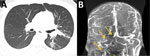
In 2021, a 25-year-old Hispanic man from New Mexico with a past medical history of poorly controlled diabetes mellitus type 2 sought care in Texas for right ear pain. Physical examination indicated swelling and tenderness of the right mastoid, along with purulent drainage from the right ear. CT of the temporal bone showed acute right otomastoiditis with thrombophlebitis of the right transverse and sigmoid dural sinuses and right internal jugular vein. The patient also had an associated venous thrombus and abscess under the right mastoid process. He underwent a right mastoidectomy and myringotomy tube placement for his abscess. The abscess cultures grew methicillin-susceptible Staphylococcus aureus and later, after the patient was discharged, Coccidioides. The patient was discharged with a prescription for intravenous nafcillin for 6 weeks and oral apixaban. However, the abscess and thrombus persisted, and he began to experience shortness of breath 1 month after discharge. He was hospitalized because of disease progression, and chest CT showed septic emboli of the left lower lung lobe and lingula (Figure 2, panel A). Given the lack of improvement, it was thought that the Coccidioides infection was playing a larger role in the disease, and intravenous liposomal amphotericin B (5 mg/kg) and oral fluconazole (800 mg/d) were initiated. The right neck abscess was incised and drained, and cultures grew Coccidioides. CF titers were 1:32. Magnetic resonance venography showed progression of the thrombus to the sagittal sinus despite anticoagulation therapy. The interventional radiologist attempted a thrombectomy, which was unsuccessful. It was determined that the thrombus progression was most likely caused by Coccidioides thrombophlebitis, and a heparin infusion was started. At 6-week follow-up, the patient was improving with no neurologic dysfunction; repeat magnetic resonance venography showed stability of the thrombus (Figure 2, panel B), and the patient was discharged with prescriptions for voriconazole and apixaban. Repeat CF titers were 1:64 and remained stable for 10 months.
With a literature search, we identified 4 additional cases of coccidioidomycosis involving the ear, mastoid bone, or both (6–8) and summarized them along with the 5 cases in our series (Table). Eight patients for whom details were available had lived in or traveled to California. Ages ranged from 4 to 76 years, and 6 of 9 were male. Ethnicity was known for 7 patients: 4 were Hispanic and 3 were White. Two patients had poorly controlled diabetes mellitus (1 each with types 1 and 2). Another patient had systemic lupus erythematosus, which had been treated with corticosteroids 1 month before the coccidioidal otomastoiditis developed. In retrospect, however, misdiagnosed coccidioidomycosis was probably the cause of the fatigue, fever, and arthralgias (with positive antinuclear and anti-DNA antibodies) attributed to lupus. Seven patients had otomastoiditis, 1 had mastoiditis without otitis, and 1 had otitis without mastoiditis. Ear pain was noted for 5 patients, mastoid swelling or tenderness for 3, and hearing loss for 2. Two patients had cutaneous lesions at the ear.
In addition to the ears or mastoids, 4 patients had previous or concomitant pulmonary disease consistent with coccidioidomycosis, including 1 patient who additionally had osteoarticular coccidioidomycosis. Ipsilateral facial nerve palsies developed in 2 patients, early for one and several months into treatment for the other. In 1 patient, coccidioidal otomastoiditis was complicated by ipsilateral internal jugular thrombosis and septic emboli.
Coccidioides species was cultured from middle ear fluid for 4 patients, mastoid tissue for 2, and both middle ear fluid and mastoid for 1. For 3 patients, diagnosis was based on histopathologic findings of spherules in mastoid tissue and for another, by histopathologic examination of skin. All 9 patients received antifungal drugs: amphotericin B for 2, amphotericin B followed by an azole for 3, and azoles alone for 4. Eight patients underwent surgical irrigation and debridement.
Coccidioidomycosis is one of the most common dimorphic fungal diseases encountered in North America; the geographic range on the continent is primarily the southwestern United States and parts of Mexico (9). Myriad manifestations have been described (4). Ear or mastoid bone involvement, however, is rare with coccidioidomycosis. In addition to the 5 cases in our series, we identified only 4 cases of ear or mastoid coccidioidomycosis in the literature. In a database of 3,000 patients with coccidioidomycosis at Kern Medical Center, only 1 case of otomycosis or otomastoiditis (case 4) was identified, highlighting the uncommon nature of this manifestation.
The mechanism by which coccidioidomycosis involves the ear or mastoid is unclear. Because those structures are contiguous with the upper respiratory tract, primary infection of the ears and mastoids after inhalation of Coccidioides arthroconidia is plausible. However, there was evidence of pulmonary disease for only 4 of 9 patients, consistent with the lack of pulmonary signs or symptoms in other patients with disseminated coccidioidomycosis involving sites not contiguous with the airways (10). Hematogenous dissemination is also a potential mechanism; Scalarone and Huntington showed in a murine model of coccidioidomycosis that intraperitoneal inoculation (which mimics hematogenous infection) with a low-virulence strain of C. immitis can lead to infection that localizes to inner ear structures and mastoids (11). It is unclear whether strain virulence or inoculation route differs among patients with this uncommon manifestation.
Although most cases of otitis or mastoiditis are bacterial, the diagnosis of coccidioidomycosis should be considered for patients with otitis or mastoiditis who have resided in or traveled to areas of risk for coccidioidomycosis, especially if the case does not respond to empiric antibacterial therapy. The diagnosis can be suggested by serologic testing, but confirmation requires culture of discharge from the ear or by biopsy of the mastoid for histopathology and fungal culture.
The optimal treatment for coccidioidomycosis involving the ear or mastoids is unknown. Most of the patients we reviewed underwent debridement, as is typically performed for fungal ear or mastoid disease. Antifungal drugs are a major component of management, although the optimal agent and duration remain unresolved. In this series, the duration of therapy ranged from 1 week (of amphotericin B) to indefinitely (suppressive azole therapy), and the optimal duration is unresolved. Repeated clinical, radiographic, and serologic evaluation with CF should be obtained before therapy is discontinued. Given the proximity and potential for invasion of the central nervous system, lifelong azole therapy should be weighed against toxicities associated with long-term use (12). Our patient series demonstrates the protean nature of coccidioidomycosis. Clinicians should consider a diagnosis of coccidioidomycosis for patients with otomastoiditis refractory to standard therapy and a history of exposure to areas of geographic risk.
Dr. Schwartz is an infectious diseases physician and researcher at Duke University School of Medicine. His research interests are emerging fungal infections, endemic mycoses, immunocompromised hosts, and global health.
Acknowledgment
G.R.T. reports grants or contracts from the National Institutes of Health, Astellas, Cidara, Mayne, F2G, Merck, Pfizer, Scynexis, and consulting fees from Astellas, Cidara, Mayne, F2G, Scynexis, all outside of the current work. All other authors declare no conflicts.
References
- Gorris ME, Treseder KK, Zender CS, Randerson JT. Expansion of coccidioidomycosis endemic regions in the United States in response to climate change. Geohealth. 2019;3:308–27. DOIPubMedGoogle Scholar
- Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis. 2016;63:e112–46. DOIPubMedGoogle Scholar
- Thompson GR III, Le T, Chindamporn A, Kauffman CA, Alastruey-Izquierdo A, Ampel NM, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:e364–74. DOIPubMedGoogle Scholar
- Bays DJ, Thompson GR III. Coccidioidomycosis. Infect Dis Clin North Am. 2021;35:453–69. DOIPubMedGoogle Scholar
- Stockamp NW, Thompson GR III. Coccidioidomycosis. Infect Dis Clin North Am. 2016;30:229–46. DOIPubMedGoogle Scholar
- Harvey RP, Pappagianis D, Cochran J, Stevens DA. Otomycosis due to coccidioidomycosis. Arch Intern Med. 1978;138:1434–5. DOIPubMedGoogle Scholar
- Busch RF. Coccidioidomycosis of the external ear. Otolaryngol Head Neck Surg. 1992;107:491–2. DOIPubMedGoogle Scholar
- Low WS, Seid AB, Pransky SM, Kearns DB. Coccidioides immitis subperiosteal abscess of the temporal bone in a child. Arch Otolaryngol Head Neck Surg. 1996;122:189–92. DOIPubMedGoogle Scholar
- Ashraf N, Kubat RC, Poplin V, Adenis AA, Denning DW, Wright L, et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020;185:843–65. DOIPubMedGoogle Scholar
- Bays DJ, Thompson GR III, Reef S, Snyder L, Freifeld AJ, Huppert M, et al. Natural history of disseminated coccidioidomycosis: examination of the Veterans Affairs–Armed Forces database. Clin Infect Dis. 2021;73:e3814–9. DOIPubMedGoogle Scholar
- Scalarone GM, Huntington RW. Circling syndrome and inner ear disease in mice infected intraperitoneally or intravenously with Coccidioides immitis spherule-endospore phase cultures. Mycopathologia. 1983;83:75–86. DOIPubMedGoogle Scholar
- Thompson GR III, Lewis JS II, Nix DE, Patterson TF. Current concepts and future directions in the pharmacology and treatment of coccidioidomycosis. Med Mycol. 2019;57(Supplement_1):S76–84. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: May 22, 2023
Table of Contents – Volume 29, Number 7—July 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ilan S. Schwartz, Duke University School of Medicine, Hanes House, 315 Trent Dr, Durham, NC 27710, USA
Top