Volume 31, Number 11—November 2025
Research Letter
Mortality Event in Rainbow Snakes Linked to Snake Fungal Disease, United States
Cite This Article
Citation for Media
Abstract
We report mortality in rainbow snakes in Virginia and North Carolina, USA, linked to snake fungal disease caused by Ophidiomyces ophidiicola. During 2013–2023, we observed 46 dead rainbow snakes with lesions indicative of snake fungal disease, noted elevated disease severity compared with other species, and recorded fewer live snakes over time.
Detecting and assessing declines in elusive or rare species can be difficult. Early identification of populations in decline can help accelerate intervention strategies and reduce the likelihood of genetic bottlenecks, population extirpation, and trophic disturbances of ecologically important species (1). Snake fungal disease (SFD) is caused by the fungal pathogen Ophidiomyces ophidiicola and affects a broad range of snake species (2), causing skin lesions as the fungus invades tissues, sometimes leading to impaired movement, anorexia, and even death (3). Researchers have documented population impacts from SFD in 2 snake species (4,5), but the extent of mortality across snake species is likely underestimated due to the cryptic nature of snakes. We describe a multiyear mortality event associated with SFD in a rare species, the rainbow snake (Farancia erytrogramma), in Virginia and North Carolina, USA.
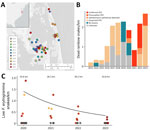
In spring 2019, regional biologists from the Back Bay region of North Carolina and Virginia reported 6 deceased rainbow snakes. In spring of 2020, we found an additional 6 snakes in the same area (Figure 1, panel A). After those events, we gathered additional records of dead rainbow snakes (Appendix Table 1) from the region and began regular surveys and sampling of rainbow snakes and other species for O. ophidiicola in 2020–2023 (Appendix).
We captured and swabbed snakes in accordance with previously published protocols (6) and used sterile procedures for disinfecting equipment (Appendix). We extracted DNA from and tested samples using quantitative PCR (qPCR) to determine the presence of O. ophidiicola, according to established methods (6,7). To quantify lesion severity for all live-captured snakes, we used an approach integrating snake size, lesion size, and lesion progression (Appendix Table 2, Figure 1).
All dead rainbow snakes screened by qPCR (n = 9) were positive for O. ophidiicola and had skin lesions characteristic of SFD (Figure 1, panel B). Necropsies on a subsample (n = 6) indicated all snakes examined had lesions consistent with O. ophidiicola infection, including thickening of the epidermis by eosinophilic necrotic cellular debris containing fungal hyphae. Snakes also had invasion by hyphae in deeper tissue, including the dermis (6/6) (Appendix Figure 2, panel A), underlying skeletal muscle (3/6), and in oral and nasal epithelium and tooth pulp (1/6). We also observed hyphae or fibrin thrombi within blood vessels in the dermis (5/6). Most snakes (4/6) were in good body condition, had large amounts of fat, and showed no signs of other serious pathologic processes. We considered SFD as the ultimate cause of death in all 6 snakes.
We also examined the number of live rainbow snakes captured over time using sampling data from standardized surveys conducted in 2020–2023 to assess preliminary trends while accounting for effort (Figure 1, panel C). We found a general decrease in the probability of detecting live rainbow snakes over time (log-scale year coefficient = −0.482 ± 0.226; p = 0.033) (Figure 1, panel C; Appendix Tables 3, 4).
A broader comparison of O. ophidiicola prevalence and severity of infection among other snakes in the community revealed that rainbow snakes were among the most infected species (Figure 2): O. ophidiicola prevalence was 80.1% (95% CIs 62.5%–92.5%), and log10 lesion severity was −1.71 (95% CI −1.94 to −1.50) (Appendix). Several other snake species also had notably high prevalence of O. ophidiicola and did not differ greatly from rainbow snakes (Figure 2, panel A) but were not found dead as part of the ongoing mortality event. Although live rainbow snakes were rare within the broader snake community (total live captures 16.4% [95% CI 11.3%–23%]; n = 25/153), they were disproportionately represented among dead snakes (87.5% [95% CI 52.9%–97.8%]; n = 7/8) found during the same period (2020–2023).
Observing wildlife mortality without obvious cause is rare and can indicate a more serious problem (8). We documented mortality of rainbow snakes using photographic, molecular, and histologic evidence, providing support that infection with O. ophidiicola is likely responsible. The rainbow snakes are considered a species of conservation concern, and although mortality in the species appeared to be ongoing as of 2023, the full extent of population declines remains uncertain.
O. ophidiicola was likely introduced to the United States in the early 1900s, although new (and possibly more virulent) strains have emerged recently (9). Increases in rainbow snake mortality could be the result of the introduction of more virulent strains of O. ophidiicola or shifts in environmental conditions since 2014 (10), but it is unclear why rainbow snakes appear particularly susceptible to infection (Figure 2). Nonetheless, the observed epizootic from a pathogen that has existed in North America for decades suggests SFD remains a threat to snake populations, which are a critical ecologic component of many ecosystems. Further research on the potential effects of O. ophidiicola would help clarify the impacts and trends of this disease on snake populations. Our study highlights the potential impact of disease-causing fungi such as O. ophidiicola on unmonitored, cryptic snake species like the rainbow snake.
Mr. Conley is a PhD student in the Department of Biological Sciences at Virginia Polytechnic Institute and State University. His primary research interests include herpetology, ecology, and infectious disease in wildlife.
Acknowledgments
We acknowledge M. Masterson and E. Molleen for their contributions in the field and A. Iyoob, J. Eagleton, C. Blake, and S. McDaniel for their observations. We also acknowledge Virginia Department of Wildlife Resources for contributing resources and support and the Virginia Department of Conservation and Recreation, North Carolina Wildlife Resource Commission, and United States Fish and Wildlife Service for granting us permission to conduct research on their properties. We also thank personnel at the US Geological Survey–National Wildlife Health Center for assistance with necropsy and laboratory testing.
Sampling of snakes for this study was approved by Virginia Tech Animal Care and Use Committee protocol #20-066. Additional metadata associated with this study that were collected by the US Geological Survey are available at https://doi.org/10.5066/P14U7ISF.
This study was financially supported by National Institutes of Health–National Science Foundation Ecology and Evolution of Infectious Disease award 1R01GM152978.
References
- Mörner T, Obendorf DL, Artois M, Woodford MH. Surveillance and monitoring of wildlife diseases. Rev Sci Tech. 2002;21:67–76. DOIPubMedGoogle Scholar
- Di Nicola MR, Coppari L, Notomista T, Marini D. Ophidiomyces ophidiicola detection and infection: a global review on a potential threat to the world’s snake populations. Eur J Wildl Res. 2022;68:64. DOIGoogle Scholar
- Lorch JM, Knowles S, Lankton JS, Michell K, Edwards JL, Kapfer JM, et al. Snake fungal disease: an emerging threat to wild snakes. Philos Trans R Soc Lond B Biol Sci. 2016;371:
20150457 . DOIPubMedGoogle Scholar - Clark RW, Marchand MN, Clifford BJ, Stechert R, Stephens S. Decline of an isolated timber rattlesnake (Crotalus horridus) population: interactions between climate change, disease, and loss of genetic diversity. Biol Conserv. 2011;144:886–91. DOIGoogle Scholar
- Tetzlaff SJ, Ravesi MJ, Allender MC, Carter ET, DeGregorio BA, Josimovich JM, et al. Snake fungal disease affects behavior of free-ranging massasauga rattlesnakes (Sistrurus catenatus). . Herpetol Conserv Biol. 2017;12:624–34.
- Blanvillain G, Lorch JM, Joudrier N, Bury S, Cuenot T, Franzen M, et al. Contribution of host species and pathogen clade to snake fungal disease hotspots in Europe. Commun Biol. 2024;7:440. DOIPubMedGoogle Scholar
- Bohuski E, Lorch JM, Griffin KM, Blehert DS. TaqMan real-time polymerase chain reaction for detection of Ophidiomyces ophiodiicola, the fungus associated with snake fungal disease. BMC Vet Res. 2015;11:95. DOIPubMedGoogle Scholar
- Smith TC, Picco AM, Knapp R. Ranaviruses infect mountain yellow-legged frogs (Rana muscosa and Rana sierrae) threatened by Batrachochytrium dendrobatidis. Herpetol Conserv Biol. 2017;12:149–59.
- Ladner JT, Palmer JM, Ettinger CL, Stajich JE, Farrell TM, Glorioso BM, et al. The population genetics of the causative agent of snake fungal disease indicate recent introductions to the USA. PLoS Biol. 2022;20:
e3001676 . DOIPubMedGoogle Scholar - Guthrie AL, Knowles S, Ballmann AE, Lorch JM. Detection of snake fungal disease due to Ophidiomyces ophiodiicola in virginia, USA. J Wildl Dis. 2016;52:143–9. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: December 04, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 11—November 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Dane Conley, Polytechnic Institute and State University, 1015 Life Science Cir, Steger 352, Blacksburg, VA 24061, USA
Top