Volume 29, Number 9—September 2023
Dispatch
Population-Based Serologic Survey of Vibrio cholerae Antibody Titers before Cholera Outbreak, Haiti, 2022
Cite This Article
Citation for Media
Abstract
A Vibrio cholerae O1 outbreak emerged in Haiti in October 2022 after years of cholera absence. In samples from a 2021 serosurvey, we found lower circulating antibodies against V. cholerae lipopolysaccharide in children <5 years of age and no vibriocidal antibodies, suggesting high susceptibility to cholera, especially among young children.
In October 2010, a United Nations peacekeeping mission to Haiti following a highly destructive earthquake inadvertently introduced cholera (1,2), leading to ≈820,000 cases and ≈10,000 deaths over the following 9 years (3). The last confirmed case from that outbreak was reported in January 2019 (4), commencing a 3-year period with no confirmed cases. Unfortunately, following a wave of sociopolitical instability that compromised sanitation, 2 cases of cholera were reported on October 2, 2022, and a new outbreak began thereafter; as of February 24, 2023, the outbreak had led to >33,000 suspected cases and 590 registered deaths (5). Phylogenetic analyses suggested the current strain descended from the Vibrio cholerae O1 Ogawa strain responsible for the original outbreak (6; C.N. Mavian et al., unpub data; http://medrxiv.org/lookup/doi/10.1101/2022.11.21.22282526). Although previous infection or vaccination can provide protective immunity, persons not exposed to cholera during the earlier outbreak would be immunologically naive and at higher risk for infection, a hypothesis supported by high reported rates of cholera in young children (5).
After exposure to V. cholerae, the predominant adaptive antibody response is to cholera toxin and lipopolysaccharide (LPS) (7). However, the most clearly defined nonmechanistic correlate of protection for cholera is presence of vibriocidal antibodies that target the O-specific antigen of the V. cholerae LPS (8,9). Circulating antibody titers peak within several weeks after infection and slowly wane to baseline over ensuing months, with high levels of variability among patients (8). Killed whole-cell oral cholera vaccines (OCVs), such as those distributed during vaccination campaigns in Haiti, are 58% effective for the first 2 years, but effectiveness declines to 26% by 4 years after vaccination (10). Children >5 years of age show ≈50% OCV protection level at 2-year follow-up compared with adults (11). Because no natural infections were reported and vaccinations were not administered during the 3 years preceding the 2022 outbreak, we investigated the presence of V. cholerae–specific antibodies in adults and children by analyzing samples collected in a cross-sectional serologic survey in 2 communes in the Ouest Department of Haiti conducted before the 2022 outbreak.
We collected dried blood spots from 861 enrolled participants, 564 adults and 297 children (<18 years of age) (Table); 62.6% were female and 37.4% male. A small percentage of participants self-reported previous cholera vaccination (1.2%; n = 10) or clinical disease (4.3%; n = 37). We performed ELISAs on all dried blood spot eluates to assess the quantity of circulating cholera toxin B (CtxB) or V. cholerae–specific LPS antibodies. For persons with IgG titers for either epitope >2 SD above the mean, we performed vibriocidal assays to assess presence of functional antibodies (Appendix).
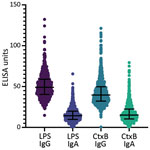
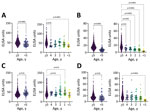
We measured antibody titers for V. cholerae LPS and CtxB for both IgG and IgA isotypes in all participants (Figure 1, panel A). Children <5 years of age had significantly lower titers of both LPS IgG and IgA compared with older children and adults (p<0.0001; Figure 2, panels A, B; Appendix Table 1). CtxB IgG was elevated in children <5 years of age (p = 0.0033), especially those 1 (p = 0.0024) or 2 (p = 0.0011) years of age (Figure 2, panel C). We found significant differences in CtxB IgA among children <5 years of age, older children, and adults, but this finding was driven by results from children <1 year of age, who may lack antibodies for reasons unrelated to V. cholerae exposure (Figure 2, panel D; Appendix Table 1) (12). Using generalized additive model–based splines, we estimated a significant positive nonlinear association of IgA isotypes with age (LPS: effective degrees of freedom [EDF] 3.4, p<0.0001; CtxB: EDF 2.0, p<0.0001) (Appendix Figure 1). This association was not significant for IgG isotypes (LPS: EDF 4.9, p = 0.12; CtxB: EDF 1.0, p = 0.13). We conducted vibriocidal assays on a subset (n = 51/861, 5.9%) of samples (Appendix), but no tested samples had detectable vibriocidal responses (Figure 1, panel B).
In this cross-sectional serologic survey within Haiti, we detected low rates of circulating IgG and IgA for LPS and CtxB. Children 1–4 years of age had lower titers of LPS IgG and IgA compared with adults and children >5 years of age. Children 1–2 years of age had elevated CtxB IgG titers, which may reflect the cross-reactive nature of CtxB antibodies with the heat-labile toxin of enterotoxigenic Escherichia coli, which has the highest force of infection among enteric pathogens among children in Haiti (13). Because of that inherent cross-reactivity for CtxB, LPS IgG is a more specific measure for history of exposure to V. cholerae, and the IgG isotype is a more meaningful for comparisons among age groups. However, we detected no vibriocidal antibodies, the best available correlate for protection against cholera.
Association of results of serologic assays used in our study with previous V. cholerae O1 infection has been shown based on longitudinal studies of culture-confirmed cholera patients (9) and with protection against disease based on studies of household contacts of index cases and in human challenge studies (8,13,14). Our data were consistent with data on limited recent disease transmission and antigenic exposure in Haiti, especially among young children born during the period in which little pandemic V. cholerae was circulating. Those serologic data suggest that persons in communities in Haiti who were serosurveyed, especially children <5 years of age, may have limited preexisting immunologic protection against cholera.
The 2022 outbreak was caused by a V. cholerae Ogawa isolate that aligns with isolates circulating during the 2010–2019 outbreak (6; C.N. Mavian et al., unpub data). The degree to which V. cholerae circulated in human and environmental reservoirs at a level below the threshold detectable by the surveillance infrastructure during the period between outbreaks is unknown. The intersection between low levels of circulating cholera and declining population immunity, combined with the collapse of clean water and sanitation infrastructure, likely put residents of Haiti at risk for cholera and led to the 2022 outbreak.
This study was limited by risk of enrollment bias because only 28% of the households screened consented to participate. Given disproportionate sampling in low population density grid cells, true distribution of cholera incidence across the population of Haiti would need to be adjusted before using these data for future serosurveillance research. In addition, observations of relatively lower IgA titers in children 1–4 years of age might have been part of a larger trend of IgA responses increasing with age, a confounding factor that might inaccurately reflect the number of specific exposures. Third, ELISA was limited by availability of quantitative and matrix-matched controls, leading us to use convalescent plasma as positive and naive serum as negative controls. Fourth, selecting samples with high ELISA units for vibriocidal assays may have missed samples with lower antibody levels that harbored functional antibodies. Finally, our serosurvey was limited to 2 adjacent communes in the Ouest Department of Haiti; hence, findings may not be generalizable to other parts of the country.
In summary, our population-based serosurvey of 2 Haitian communities revealed a lack of functional antibodies and significantly lower V. cholerae LPS–specific IgG among young children than older children and adults. These findings suggest persons, especially young children, in Haiti may have high susceptibility to cholera cases and outbreaks.
Dr. Clutter is a postdoctoral fellow at the University of Utah investigating cholera seroepidemiology and adaptive immunity during metabolic disease. Ms. Klarman is director of research initiatives at the University of Florida Public Health Research Lab in Gressier, Haiti. Initiatives include surveillance of emerging diseases and efforts to improve early access to healthcare for preemergency patients in resource-limited settings.
Acknowledgments
We are grateful to the participants as well as the team who conducted the survey in Haiti. We would like to thank the administrators and leadership at the Emerging Pathogens Institute and the Department of Pediatrics at the University of Florida for their ongoing support. We also honor the dedication and support of the Ministry of Public Health and Population (Ministère de la Santé Publique et de la Population) for their commitment to the health of the Haitian population despite the profound period of instability.
Funding for this study was provided through grant support to D.T.L. from the National Institute of Allergy and Infectious Diseases (R01 AI135115) and to E.J.N. from the Children’s Miracle Network (Florida, USA). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, et al. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364:33–42. DOIPubMedGoogle Scholar
- Frerichs RR, Keim PS, Barrais R, Piarroux R. Nepalese origin of cholera epidemic in Haiti. Clin Microbiol Infect. 2012;18:E158–63. DOIPubMedGoogle Scholar
- World Health Organization. Cholera—Haiti [cited 2023 Feb 7]. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON427
- World Health Organization. Cholera, 2019. Wkly Epidemiol Rec. 2020;95:441–8.
- Pan American Health Organization; World Health Organization. Epidemiological update—cholera—28 February 2023 [cited 2023 Feb 7] https://www.paho.org/en/documents/epidemiological-update-cholera-28-february-2023
- Rubin DHF, Zingl FG, Leitner DR, Ternier R, Compere V, Marseille S, et al. Reemergence of Cholera in Haiti. N Engl J Med. 2022;387:2387–9. DOIPubMedGoogle Scholar
- Kauffman RC, Bhuiyan TR, Nakajima R, Mayo-Smith LM, Rashu R, Hoq MR, et al. Single-cell analysis of the plasmablast response to Vibrio cholerae demonstrates expansion of cross-reactive memory B cells. MBio. 2016;7:e02021–16. DOIPubMedGoogle Scholar
- Azman AS, Lessler J, Luquero FJ, Bhuiyan TR, Khan AI, Chowdhury F, et al. Estimating cholera incidence with cross-sectional serology. Sci Transl Med. 2019;11:
eaau6242 . DOIPubMedGoogle Scholar - Iyer AS, Harris JB. Correlates of protection for cholera. J Infect Dis. 2021;224(Suppl 2):S732–7. DOIPubMedGoogle Scholar
- Bi Q, Ferreras E, Pezzoli L, Legros D, Ivers LC, Date K, et al.; Oral Cholera Vaccine Working Group of The Global Task Force on Cholera Control. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1080–8. DOIPubMedGoogle Scholar
- Qadri F, Wierzba TF, Ali M, Chowdhury F, Khan AI, Saha A, et al. Efficacy of a single-dose, inactivated oral cholera vaccine in Bangladesh. N Engl J Med. 2016;374:1723–32. DOIPubMedGoogle Scholar
- Arnold BF, Martin DL, Juma J, Mkocha H, Ochieng JB, Cooley GM, et al. Enteropathogen antibody dynamics and force of infection among children in low-resource settings. eLife. 2019;8:
e45594 . DOIPubMedGoogle Scholar - Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque ASG, et al. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2:
e221 . DOIPubMedGoogle Scholar - Haney DJ, Lock MD, Simon JK, Harris J, Gurwith M. Antibody-based correlates of protection against cholera analysis of a challenge study in a cholera-naive population. Clin Vaccine Immunol. 2017;24:e00098–17. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: July 24, 2023
1These first authors contributed equally to this article.
2These senior authors contributed equally to this article.
Table of Contents – Volume 29, Number 9—September 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Christy H. Clutter, Department of Internal Medicine, Division of Infectious Disease, University of Utah, Wintrobe Research Building, Rm 517, 26 North Medical Dr N, Salt Lake City, UT 84112, USA
Top