Volume 30, Number 11—November 2024
Research
Risk for Facial Palsy after COVID-19 Vaccination, South Korea, 2021–2022
Figure 2
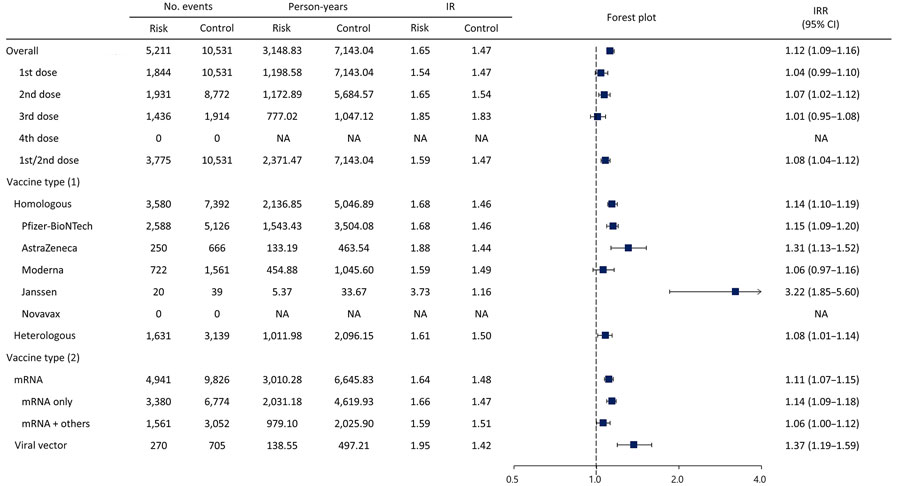
Figure 2. Forest plot of risk for facial palsy after COVID-19 vaccination in South Korea, 2021–2022. Plot assess facial palsy risk within 28 days of COVID-19 vaccination. Overall risk is shown, as is risk stratified by dose and vaccine type. Squares indicate IRRs; bars indicate 95% CIs. Vaccine types were BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com), mRNA-1273 (Moderna, https://www.modernatx.com), ChAdOx1 nCoV-19 (AstraZeneca, https://www.astrazeneca.com), Ad.26.COV2.S (Janssen, https://www.janssen.com), and NVX-CoV2373 (Novavax, https://www.novavax.com). 1st/2nd dose indicates a first dose of BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, or Ad26.COV2.S and a second dose of BNT162b2, mRNA-1273, or ChAdOx1 nCoV-19. IR, incidence rate; IRR, incidence rate ratio; NA, not applicable.
1These first authors contributed equally to this article.
2These last authors contributed equally to this article.