Volume 30, Number 11—November 2024
Research Letter
Iquitos Virus in Traveler Returning to the United States from Ecuador
Cite This Article
Citation for Media
Abstract
We describe the case of a returned traveler to the United States from Ecuador who had an acute febrile illness, initially diagnosed as Oropouche fever. This illness was later confirmed to be a rare infection with Iquitos virus, a related bunyavirus that shares 2 of 3 genome segments with Oropouche virus.
Oropouche virus (OROV) is a species in the Simbu serogroup of bunyaviruses (genus Orthobunyavirus) that includes the reassortant species Iquitos virus (IQTV) and Madre de Dios virus. The species share common small and large genome segments but differ in the medium segment (1,2). Symptomatic human infections with the viruses typically manifest with nonspecific signs and symptoms (e.g., fever, headache, myalgias, and arthralgias) that cannot be clinically differentiated from other common tropical febrile illnesses such as dengue, malaria, and leptospirosis (2–4).
In 2024, a large OROV outbreak has affected countries of the Amazon River Basin, with cases occurring in Brazil, Peru, Colombia, and Bolivia (5). However, despite previous detection of OROV transmission in Ecuador in 2016–2017 (3), cases have not been reported to the Pan American Health Organization from Ecuador in 2024 (5).
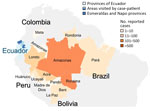
In early April 2024, a 38-year-old man with no notable medical history visited a healthcare system in Atlanta, Georgia, USA, with an acute febrile illness after returning from Ecuador. During a 10-day itinerary, he visited the capital city of Quito, Esmeraldas Province in the northwest, and Napo Province in the Amazon Basin (Figure 1), where he noted numerous bug bites. The patient did not take malaria prophylaxis but consistently used a DEET-containing insect repellant during the trip. He had 1 day of diarrhea on his last day in Ecuador; then, 2 days after returning to the United States, he had fevers reaching 102°F and chills, sweats, headache, and pain with eye movement. He was seen in an emergency department and was found to have a slight elevation in his creatinine (1.3 mg/dL [reference range 0.6-1.2 mg/dL]) but otherwise normal complete metabolic panel and complete blood count results. He was discharged home with an outpatient referral to the Emory TravelWell Center (also in Atlanta) the following day.
During that visit, he reported fatigue, sleeping 12–14 hours a day, and numerous itchy “bug bites” on his arms and ankles. Examination revealed he had unremarkable vital signs and a diffuse papular rash across his forearms and lower legs. The patient’s illness was reported in the GeoSentinel database (6), and he consented to the collection and testing of EDTA whole blood and serum as part of a research study into the causes of infection in returned travelers. At a follow-up visit the next week, he reported that the fever had resolved after 2 days and that the headache and fatigue resolved over 10 days. The rash initially evolved to hyperpigmented plaques and then resolved with topical hydrocortisone and diphenhydramine.
We processed whole blood and serum with a laboratory-developed nucleic acid extraction and storage protocol (i.e., the RNA extraction and storage [RNAES] protocol) (7). All eluates were negative for Zika, chikungunya, and dengue viruses on a laboratory-developed assay and negative for Leptospira and Plasmodium species (8). Eluates from serum and whole blood tested positive in a laboratory-developed real-time reverse transcription PCR (RT-PCR) that targets the small genome segment of OROV and related bunyaviruses (Appendix Figure, panel A) (4). We confirmed this finding by reextraction and retesting of an aliquot of whole blood using a second real-time RT-PCR targeting a different portion of the small genome segment (Appendix Figure, panel B) (9).
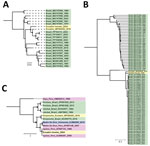
We successfully generated partial sequences for the coding regions of the small (83%), medium (27%), and large (37%) segments (GenBank accession nos. PQ325301–4) (Appendix). Phylogenetic analysis indicated that the small and large segments from the returned traveler were most closely related to an Oropouche virus sample obtained in Ecuador in 2016, and they clustered just basal to sequences from samples obtained from Brazil in 2023 (Figure 2, panel A, B). However, phylogenetic analysis of the medium segment confirmed that it was most closely related to IQTV, the only other available sequences of which were from Peru (Figure 2, panel C).
Fever in a returned traveler can result from myriad etiologies that may be unfamiliar to providers in nonendemic areas and for which diagnostic testing is often limited (6). For the case we describe, systematic screening tools and economical laboratory solutions enabled the initial detection of OROV or a related bunyavirus, which has important implications for clinical management, given that meningitis and relapsing disease have been reported in OROV infection (2). However, further characterization by next-generation sequencing identified this virus as IQTV, a related bunyavirus that also circulates in the Amazon Basin and may have contributed to reassortment events that led to current OROV genetic diversity in South America (10). IQTV reportedly causes a clinical illness similar to Oropouche fever; of note, however, infection with OROV does not appear to protect against future IQTV infection (1). Finally, this case provides support for increased bunyavirus monitoring in Ecuador (3), where these viruses may have gone undetected or underreported because of limited diagnostics, poor healthcare access, sociopolitical instability, or a combination of those factors.
Ms. Baer is a physician assistant at the Emory TravelWell Center. Her primary research centers on improving medical care for international travelers and migrants. Dr. Arora is an associate bioinformatics scientist in the Department of Infectious Diseases at Emory University School of Medicine. Her primary research interests are developing bioinformatics pipelines for next-generation sequencing to study emerging viruses.
Acknowledgments
We appreciate the patient’s willingness to participate in this study and the technical and clinical staff who made this possible.
New sequences generated for this study have been uploaded into GenBank and have the following accession numbers: small segment, PQ325301; medium segment, PQ325302 and PQ325303; large segment, PQ325304.
This work was supported by the Georgia Research Alliance (grant no. GRA.VL24.C4), a pilot project grant from the International Society of Travel Medicine, the National Institutes of Health’s National Institute of Allergy and Infectious Diseases–funded Centers for Research in Emerging Infectious Diseases Network (awarded to the American and Asian Centers for Arboviral Research and Enhanced Surveillance Center under grant no. U01AI151788-03), the Center for the Advancement of Diagnostics for a Just Society based at Emory University, and the Centers for Disease Control and Prevention–funded Georgia Pathogen Genomics Center of Excellence.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Advancement of Diagnostics for a Just Society Center, Emory University, or the Centers for Disease Control and Prevention.
References
- Aguilar PV, Barrett AD, Saeed MF, Watts DM, Russell K, Guevara C, et al. Iquitos virus: a novel reassortant Orthobunyavirus associated with human illness in Peru. PLoS Negl Trop Dis. 2011;5:
e1315 . DOIPubMedGoogle Scholar - Sakkas H, Bozidis P, Franks A, Papadopoulou C. Oropouche fever: a review. Viruses. 2018;10:175. DOIPubMedGoogle Scholar
- Wise EL, Pullan ST, Márquez S, Paz V, Mosquera JD, Zapata S, et al. Isolation of Oropouche virus from febrile patient, Ecuador. Emerg Infect Dis. 2018;24:935–7. DOIPubMedGoogle Scholar
- Rojas A, Stittleburg V, Cardozo F, Bopp N, Cantero C, López S, et al. Real-time RT-PCR for the detection and quantitation of Oropouche virus. Diagn Microbiol Infect Dis. 2020;96:
114894 . DOIPubMedGoogle Scholar - Pan American Health Organization. Epidemiological update: Oropouche in the Americas Region. 2024 Sep 6 [cited 2024 Sep 16]. https://www.paho.org/en/documents/epidemiological-update-oropouche-americas-region-6-september-2024
- Brown AB, Miller C, Hamer DH, Kozarsky P, Libman M, Huits R, et al. Travel-related diagnoses among U.S. nonmigrant travelers or migrants presenting to U.S. GeoSentinel sites—GeoSentinel Network, 2012–2021. MMWR Surveill Summ. 2023;72:1–22. DOIPubMedGoogle Scholar
- Hernandez S, Cardozo F, Myers DR, Rojas A, Waggoner JJ. Simple and economical RNA extraction and storage packets for viral detection from serum or plasma. Microbiol Spectr. 2022;10:
e0085922 . DOIPubMedGoogle Scholar - Waggoner JJ, Abeynayake J, Balassiano I, Lefterova M, Sahoo MK, Liu Y, et al. Multiplex nucleic acid amplification test for diagnosis of dengue fever, malaria, and leptospirosis. J Clin Microbiol. 2014;52:2011–8. DOIPubMedGoogle Scholar
- Weidmann M, Rudaz V, Nunes MR, Vasconcelos PF, Hufert FT. Rapid detection of human pathogenic orthobunyaviruses. J Clin Microbiol. 2003;41:3299–305. DOIPubMedGoogle Scholar
- Naveca FG, de Almeida TAP, Souza V, Nascimento V, Silva D, Nascimento F, et al. Emergence of a novel reassortant Oropouche virus in the Brazilian Amazon region. Nat Med. 2024; [Epub ahead of print]. DOIGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: October 17, 2024
1These first authors contributed equally to this article.
Table of Contents – Volume 30, Number 11—November 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jesse J. Waggoner, Emory University School of Medicine, 1760 Haygood Dr NE, Rm E-132, Atlanta, GA 30322, USA
Top