Volume 30, Number 12—December 2024
Dispatch
Feline Panleukopenia Virus in a Marsican Brown Bear and Crested Porcupine, Italy, 2022–2023
Cite This Article
Citation for Media
Abstract
The virus species Protoparvovirus carnivoran 1 encompasses pathogens that infect both domestic and wild carnivores, including feline panleukopenia virus. We identified and characterized feline panleukopenia virus strains in a Marsican brown bear (Ursus arctos marsicanus) and a crested porcupine (Hystrix cristata) in Italy, extending the known host range of this virus.
Parvoviruses (genus Protoparvovirus, family Parvoviridae) are small, nonenveloped, single-stranded DNA viruses ≈4.5–5.5-kb long. Their linear DNA genome contains 2 major open reading frames encoding for 2 nonstructural proteins (1 and 2) and 2 capsid proteins (VP1 and VP2). Feline panleukopenia virus (FPV) and the closely related canine parvovirus (CPV or CPV-2), included in the species Protoparvovirus carnivoran 1, are highly contagious pathogens that cause acute and often fatal diseases in domestic and wild felids and canids (1).
FPV and CPV are closely related genetically and antigenically but differ in their host range and pathogenicity. The biological differences are determined by a few amino acid mutations in the VP2 capsid protein that modulate the ability to bind to cellular transferrin receptor type 1 (2). Parvoviruses have long been considered to be species specific; however, FPV and CPV-2 variants (i.e., 2a, 2b, and 2c) have been reported in a wide variety of wild carnivores. Of note, some species (e.g., cats, foxes, badgers, and giant pandas) may be infected by both FPV and CPV strains (3). We conducted a molecular investigation for Protoparvovirus carnivoran 1 viruses in wildlife in Italy.
During January 2022–May 2023, we collected tissue samples by convenience sampling from 89 animals: 44 wolves (Canis lupus), 26 red foxes (Vulpes vulpes), 15 European badgers (Meles meles), 2 stone martes (Martes foina), 1 Marsican brown bear (Ursus arctos marsicanus), and 1 crested porcupine (Hystrix cristata). All animals were found already dead in the regions of Abruzzo and Molise, Italy, and sample collection was conducted during routine necropsy procedures at Istituto Zooprofilattico Sperimentale of Abruzzo and Molise ‘Giuseppe Caporale.’ All tissues were temporarily stored at −20°C before being transported by cold chain to the Infectious Diseases Unit of the Department of Veterinary Medicine, University of Bari, Bari, Italy.
We homogenized the tissues (10% wt/vol) in Dulbecco’s modified Eagle medium and extracted viral DNA from the supernatant of the homogenates of all samples by using the IndiSpin Pathogen Kit (Indical Bioscience GmbH, https://www.indical.com), according to the manufacturer’s instructions. We used quantitative PCR (qPCR) to screen DNA extracts for the presence of CPV/FPV DNA (4) and further characterized positive samples by qPCR based on minor groove binder able to differentiate CPV types 2a/2b and 2b/2c and CPV/FPV, as described previously (4).
Overall, 52 (58.4%) of 89 samples tested positive in the qPCR screening for CPV/FPV DNA (Table 1) with a geometric mean value of 8.91 × 103 copies of parvovirus DNA/10 μL of template (range 8.48 × 100 to 5.71 × 106 DNA copies/10 μL of template), including the samples from the Marsican brown bear and the crested porcupine. We characterized the bear and porcupine viruses as FPV by using the minor groove binder qPCR with viral loads of 8.40 × 105 (bear) and 4.43 ×105 (porcupine) DNA copies/10 μL of template.
To acquire the complete viral genome sequence of the FPV strains from the 2 animals, we designed 2 multiplex PCR protocols amplifying 15 PCR-tiling amplicons of 388–511 bp (Table 2), following an ARTIC-like strategy (5). We designed primer pairs based on the consensus sequence of Protoparvovirus carnivoran1 genomes recovered from GenBank. We performed PCR by using TaKaRa La Taq polymerase (Takara Bio Europe, https://www.takarabio.com). We used 500 ng of equimolar pooled PCR products for each FPV-positive sample as the input to the libraries prepared by using Ligation Sequencing Kit (SQK-LSK110; Oxford Nanopore Technologies, https://nanoporetech.com), according to the manufacturer’s guidelines. We sequenced the libraries independently by using flongle flowcell FLO-FLG001, R9.4.1, adapted in a MinION Mk1C platform (Oxford Nanopore Technologies) for 24 hours each. We subjected FastQ MinION files to quality control, trimming, and reference assembly by using Minimap2 plugin implemented in Geneious Prime software v.2021.2.2 (Biomatters Ltd., https://www.geneious.com).
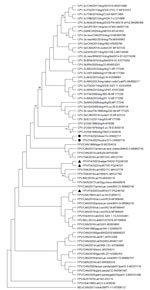
We generated the complete coding regions of the genomes for FPV strains ITA/2023/bear/74 (submitted to GenBank under accession no. OR602717) and ITA/2023/hystrix/213 (submitted to GenBank under accession no. OR602718). Sequence alignment of the complete VP2 genomic region revealed 2 aa mutations, 103-Val to Ala in both capsid sequences and 232-Val to Ile in the porcupine capsid, which are 2 key residues defining the biological properties and host range of CPV and FPV (2). VP2 residues 103- and 232- are Val in reference FPV strains, and they are replaced by Ala and Ile in CPV-2 strains. Phylogenetic analysis of the complete amino acid sequence of the VP2 gene indicated that the 2 FPV strains ITA/2023/bear/74 and ITA/2023/hystrix/213 were segregated together but separately from other FPV and CPV strains. Of note, sequencing of the FPV strains detected in other species (wolf and badger) evidenced circulation of >3 different FPV subclades/strains in the sampled animals (Figure). Also, based on our results and the geographic areas where the samples were collected, we can hypothesize that there could be an epidemiologic connection via either direct/indirect contact between porcupines and bears or other cohabiting wild animal species.
Immunofluorescence analysis of the mesenteric lymph node tissues of the crested porcupine detected parvovirus antigens (Appendix Figure). However, immunofluorescence analysis conducted on the bear samples did not allow correct interpretation because of advanced postmortem degradation of the intestinal tissue.
Serologic studies performed for several bear species (members of the family Ursidae) have shown evidence of exposure to FPV/CPV (6–8). Two serologic studies involving Marsican brown bears reported seroprevalences as high as 40% (7) and 100% (8) for FPV/CPV, although those tests do not differentiate between CPV-2 and FPV. The possibility that FPV and CPV can infect bears has also been confirmed by detection and molecular characterization of FPV and CPV-2a from giant pandas in China (9,10). In both of those instances, the VP2-coding region presented unusual amino acid mutations (i.e., 299-Gly to Glu in the FPV virus and 370-Arg to Gln in the CPV-2a strain). Whether those unique amino acid variations affected the host range of the parvovirus strains remains unknown.
A case report from 1984 in Canada described a suspected parvovirus infection in porcupines that exhibited clinical signs suggestive of parvoviral infection (11). However, electron microscopy, serologic tests, and virologic investigations did not confirm the etiology.
Recently, several new parvoviruses (e.g., bocaparvoviruses and bufaviruses) have been discovered in domestic and wild animals (12). CPV and FPV seem to be endemic to wildlife populations and have shown continuous evolution over the years; CPV/FPV-like strains have emerged with a broadened host range (3,13). Domestic pets are regarded as a source of FPV and CPV strains for wildlife carnivores through spillover infections (3,14). However, wildlife hosts may also serve as virus reservoirs and sources of infection for the domestic animal population in a perpetual cycle involving 2-way transmission among susceptible hosts. In our study, prevalence of CPV and FPV virus in wildlife was higher than expected (3,15). Genome sequencing of FPV strains detected from nonconventional FPV/CPV hosts, the Marsican brown bear and the crested porcupine, revealed amino acid mutations in key residues controlling host range and receptor affinity. Our detection of FPV strains in a Marsican brown bear and in a crested porcupine in Italy extends the known host range of this virus.
Dr. Diakoudi is a researcher at the University of Bari, Bari, Italy. Her research interests cover virus discovery in animals, with particular focus on viruses with zoonotic potential.
Acknowledgments
This research was supported by European Union funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (project no. PE00000007, INF-ACT) and by the National Laboratory for Infectious Animal Diseases, Antimicrobial Resistance, Veterinary Public Health and Food Chain Safety, RRF-2.3.1-21-2022-00001.
The authors declare no conflicts of interest in the study.
References
- Cotmore SF, Agbandje-McKenna M, Canuti M, Chiorini JA, Eis-Hubinger AM, Hughes J, et al.; Ictv Report Consortium. ICTV virus taxonomy profile: Parvoviridae. J Gen Virol. 2019;100:367–8. DOIPubMedGoogle Scholar
- Callaway HM, Welsch K, Weichert W, Allison AB, Hafenstein SL, Huang K, et al. Complex and dynamic interactions between parvovirus capsids, transferrin receptors, and antibodies control cell infection and host range. J Virol. 2018;92:e00460–18. DOIPubMedGoogle Scholar
- Ndiana LA, Lanave G, Desario C, Berjaoui S, Alfano F, Puglia I, et al. Circulation of diverse protoparvoviruses in wild carnivores, Italy. Transbound Emerg Dis. 2021;68:2489–502. DOIPubMedGoogle Scholar
- Decaro N, Desario C, Lucente MS, Amorisco F, Campolo M, Elia G, et al. Specific identification of feline panleukopenia virus and its rapid differentiation from canine parvoviruses using minor groove binder probes. J Virol Methods. 2008;147:67–71. DOIPubMedGoogle Scholar
- Quick J, Grubaugh ND, Pullan ST, Claro IM, Smith AD, Gangavarapu K, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc. 2017;12:1261–76. DOIPubMedGoogle Scholar
- Dunbar MR, Cunningham MW, Roof JC. Seroprevalence of selected disease agents from free-ranging black bears in Florida. J Wildl Dis. 1998;34:612–9. DOIPubMedGoogle Scholar
- Marsilio F, Tiscar PG, Gentile L, Roth HU, Boscagli G, Tempesta M, et al. Serologic survey for selected viral pathogens in brown bears from Italy. J Wildl Dis. 1997;33:304–7. DOIPubMedGoogle Scholar
- Di Francesco CE, Gentile L, Di Pirro V, Ladiana L, Tagliabue S, Marsilio F. Serologic evidence for selected infectious diseases in Marsican brown bears (Ursus arctos marsicanus) in Italy (2004-09). J Wildl Dis. 2015;51:209–13. DOIPubMedGoogle Scholar
- Yi S, Liu S, Meng X, Huang P, Cao Z, Jin H, et al. Feline panleukopenia virus With G299E substitution in the VP2 protein first identified from a captive giant panda in China. Front Cell Infect Microbiol. 2022;11:
820144 . DOIPubMedGoogle Scholar - Guo L, Yang SL, Chen SJ, Zhang Z, Wang C, Hou R, et al. Identification of canine parvovirus with the Q370R point mutation in the VP2 gene from a giant panda (Ailuropoda melanoleuca). Virol J. 2013;10:163. DOIPubMedGoogle Scholar
- Frelier PF, Leininger RW, Armstrong LD, Nation PN, Povey RC. Suspected parvovirus infection in porcupines. J Am Vet Med Assoc. 1984;185:1291–4.PubMedGoogle Scholar
- Jager MC, Tomlinson JE, Lopez-Astacio RA, Parrish CR, Van de Walle GR. Small but mighty: old and new parvoviruses of veterinary significance. Virol J. 2021;18:210. DOIPubMedGoogle Scholar
- Hoelzer K, Parrish CR. The emergence of parvoviruses of carnivores. Vet Res. 2010;41:39. DOIPubMedGoogle Scholar
- Behdenna A, Lembo T, Calatayud O, Cleaveland S, Halliday JEB, Packer C, et al. Transmission ecology of canine parvovirus in a multi-host, multi-pathogen system. Proc Biol Sci. 1899;2019:286.PubMedGoogle Scholar
- Miranda C, Santos N, Parrish C, Thompson G. Genetic characterization of canine parvovirus in sympatric free-ranging wild carnivores in Portugal. J Wildl Dis. 2017;53:824–31. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: November 18, 2024
Table of Contents – Volume 30, Number 12—December 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nicola Decaro, Department of Veterinary Medicine, University of Bari, Italy, S.p. per Casamassima Km 3, 70010, Valenzano, Bari, Italy
Top