Volume 30, Number 5—May 2024
Research Letter
Novel Patterns in High-Resolution Computed Tomography in Whipple Pneumonia
Cite This Article
Citation for Media
Abstract
With the use of metagenomic next-generation sequencing, patients diagnosed with Whipple pneumonia are being increasingly correctly diagnosed. We report a series of 3 cases in China that showed a novel pattern of movable infiltrates and upper lung micronodules. After treatment, the 3 patients recovered, and lung infiltrates resolved.
Whipple pneumonia is a rare, chronic, multiorgan disease, with symptoms including arthritis, diarrhea, and weight loss. Diagnosis is traditionally confirmed by a histologic examination of a small bowel biopsy (1). The causative pathogen is Tropheryma whipplei bacteria, initially identified from the aortic valve of an endocarditis patient in 2000 (2). The bacterium was successfully cultured again in 2012 by using a sample of bronchoalveolar lavage fluid (BALF) from a pneumonia patient with an acute pulmonary infection (2). By using special culture systems, laboratories can grow positive staining or immunofluorescence detectable bacteria within a macrophage or fibroblast cell in 40–60 days. Metagenomic next-generation sequencing (mNGS) is a useful tool for diagnosis.
We report 3 patients in China diagnosed with T. whipplei pneumonia by using BALF mNGS (Vision Medicals Company, http://www.visionmedicals.com) screening during July 2021–December 2022. The patients had unique radiologic patterns, including upper lung gathering of micronodules forming a galaxy sign, and slightly movable infiltrates before, during, and after treatment.
Patient 1 was a 46-year-old man with a productive cough and a 5-year history of lung abnormality. His lung lesions gradually increased over time, and we found gathering micronodules forming a galaxy sign on the right upper lung (Appendix Figure 1). T. whipplei bacteria was the only pathogen we recovered from BALF screened by using mNGS.
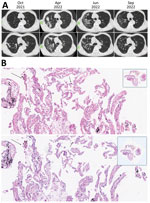
Patient 2 was a 67-year-old man with progressive dyspnea, productive cough, poor appetite, and weight loss. Repeated high-resolution computed tomography (CT) showed gradual increase of diffused micronodules gathering on the upper right lung for 6 months before diagnosis (Figure, panel A). Lesions in the upper right lung also showed movement. After bronchoscopic examination, T. whipplei bacteria was the only pathogen we recovered from BALF. Our histologic examination of the lung biopsy showed increased foamy macrophages within the alveolar space and thickened alveolar septal (Figure, panel B); neutrophils were the predominant cell type seen.
Patient 3 was a 57-year-old man with complaints of cough and chest tightness. We found diffuse ground-glass micronodules in the left upper lung (Appendix Figure 2). We performed mNGS of BALF and found Cryptococcus spp. yeast and T. whipplei bacteria. We treated the patient with fluconazole. Six months later, the patient was readmitted to our hospital because of chest tightness and dry cough. We repeated mNGS, and T. whipplei bacteria was the only pathogen identified.
The lung tissue from all 3 patients was negative for periodic acid-Schiff and anti-acid staining. We performed an enteroscopic examination on the patients 2 and 3; both were negative. We treated the 3 patients with intravenous ceftriaxone (2 g/d) for 2 weeks, then we began combination therapy of minocycline and hydroxychloroquine for an extended period. All 3 patients responded well to treatment, and chest CT showed improvement of lung lesions.
We conducted a literature review for similar cases. We systematically reviewed PubMed for “T. whipplei” or “Whipple’s disease” and “pneumonia” for the period July 2021–December 2022. We included literature for analysis if they provided individual patient and imaging data. We defined acute pulmonary infection by classic clinical manifestation and opacity on a chest radiograph or a CT scan. A total of 97 patients with Whipple pneumonia were mentioned. CT findings were available for 14 patients from 7 studies (2–8). The CT findings included bilateral alveolar consolidation, mass, nodule with cavitation, ground-glass opacity, and diffuse micronodules (Table). Mediastinal lymphadenopathy was described in 1 patient. Therapeutic outcomes were described in 5 patients, and no patients died from pneumonia. Only 1 patient had a comparative chest CT before and after treatment. No patients demonstrated movable lung infiltrates.
A 17-year-long retrospective study identified 36 patients with positive PCR results of T. whipplei bacteria; of those, 8 patients had pulmonary involvement, and only 3 patients had abnormalities in chest imaging (9). Another study showed that 6.1% (88/1,430 samples) of BALF samples were positive for T. whipplei bacteria; 58 patients had pneumonia, and T. whipplei bacteria was identified as the causative pathogen for 9 patients (10). One study analyzed the characteristics of 70 patients positive for T. whipplei bacteria in BALF detected by mNGS in which T. whipplei was the only pathogen recovered for 20 patients (8); in that study, 15 patients received therapy, and 6 patients improved after treatment (8). In our study, T. whipplei bacteria was the only pathogen in 2 patients and was repeatedly detected in the third patient. In our patients, the infiltrates exhibited movable changes over time before, during, and after treatments. Histologic examination of case 2 showed a collagen and carbon deposition within lung tissue without any history of coal mine exposure, suggesting that T. whipplei bacterial infection can cause chronic infection and scar formation, eventually leading to granulomatous-like changes within lung tissue. All 3 patients symptoms improved after receiving the first-line treatment recommendation of minocycline and hydroxychloroquine (1).
In conclusion, Whipple pneumonia is increasingly recognized when mNGS is used. We report a relatively unique feature of CT findings in patients with Whipple pneumonia and provide support for choosing combination treatment using minocycline and hydroxychloroquine.
Dr. Li is a respiratory diseases specialist at the Seventh Affiliated Hospital, Sun Yatsen University. Her research interests include the mechanisms of respiratory infection and lung injury.
References
- Boumaza A, Ben Azzouz E, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22:e280–91. DOIPubMedGoogle Scholar
- Fenollar F, Ponge T, La Scola B, Lagier JC, Lefebvre M, Raoult D. First isolation of Tropheryma whipplei from bronchoalveolar fluid and clinical implications. J Infect. 2012;65:275–8. DOIPubMedGoogle Scholar
- Stein A, Doutchi M, Fenollar F, Raoult D. Tropheryma whipplei pneumonia in a patient with HIV-2 infection. Am J Respir Crit Care Med. 2013;188:1036–7. DOIPubMedGoogle Scholar
- Zhang WM, Xu L. Pulmonary parenchymal involvement caused by Tropheryma whipplei. Open Med (Wars). 2021;16:843–6. DOIPubMedGoogle Scholar
- Kelly CA, Egan M, Rawlinson J. Whipple’s disease presenting with lung involvement. Thorax. 1996;51:343–4. DOIPubMedGoogle Scholar
- Li W, Zhang Q, Xu Y, Zhang X, Huang Q, Su Z. Severe pneumonia in adults caused by Tropheryma whipplei and Candida sp. infection: a 2019 case series. BMC Pulm Med. 2021;21:29. DOIPubMedGoogle Scholar
- Canessa PA, Pratticò L, Sivori M, Magistrelli P, Fedeli F, Cavazza A, et al. Acute fibrinous and organising pneumonia in Whipple’s disease. Monaldi Arch Chest Dis. 2008;69:186–8.PubMedGoogle Scholar
- Lin M, Wang K, Qiu L, Liang Y, Tu C, Chen M, et al. Tropheryma whipplei detection by metagenomic next-generation sequencing in bronchoalveolar lavage fluid: A cross-sectional study. Front Cell Infect Microbiol. 2022;12:
961297 . DOIPubMedGoogle Scholar - Duss FR, Jaton K, Vollenweider P, Troillet N, Greub G. Whipple disease: a 15-year retrospective study on 36 patients with positive polymerase chain reaction for Tropheryma whipplei. Clin Microbiol Infect. 2021;27:910.e9–13. DOIPubMedGoogle Scholar
- Lagier JC, Papazian L, Fenollar F, Edouard S, Melenotte C, Laroumagne S, et al. Tropheryma whipplei DNA in bronchoalveolar lavage samples: a case control study. Clin Microbiol Infect. 2016;22:875–9. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: April 10, 2024
Table of Contents – Volume 30, Number 5—May 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hui Li, the Seventh Affiliated Hospital, Sun Yatsen University, 628 Zhenyuan Rd., Shenzhen, China
Top