Volume 30, Number 6—June 2024
Dispatch
Choanephora infundibulifera Rhinosinusitis in Man with Acute Lymphoblastic Leukemia, Tennessee, USA
Cite This Article
Citation for Media
Abstract
Choanephora infundibulifera is a member of the Mucorales order of fungi. The species is associated with plants as a saprophyte or parasite and may be responsible for spoilage or disease but is an uncommon cause of human infection. We describe C. infundibulifera rhinosinusitis in a young man with leukemia in Tennessee, USA.
An 18-year-old man visited St. Jude Children Research Hospital in Memphis, Tennessee, USA, with systemic symptoms and lymphadenopathy and received a diagnosis of early T-cell precursor acute lymphoblastic leukemia. Induction chemotherapy was complicated by rhinosinusitis linked to species of Alternaria and Curvularia and presumed fungal pneumonia. The man’s treatment consisted of debridement of his nasal and sinus passages and administration of liposomal amphotericin B, followed by oral posaconazole for 5 months. Thereafter, posaconazole secondary prophylaxis was prescribed during severe neutropenia.
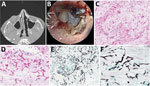
The man’s leukemia relapsed 6 months after his original diagnosis, and he received treatment that included cyclophosphamide, vincristine, doxorubicin, methotrexate, cytarabine, dexamethasone, dasatinib, and venetoclax. At 4 months postrelapse, while receiving posaconazole prophylaxis (300 mg orally 2×/d), the patient sought medical treatment for acute right facial pain and a black eschar on his anterior nasal septum. His leukocyte count was 0.16 × 103 cells/mm3 (normal 4.5 to 11.0 x 103 cells/mm3), and his absolute neutrophil count was 20 cells/mm3 (normal 1,500 to 8,000 cells/mm3). Measurement of his serum posaconazole trough concentration revealed a level of 1.4 μg/mL (desired concentration ≥0.7 μg/mL). Computed tomography of the sinuses showed evidence of rhinosinusitis (Figure 1, panel A). A magnetic resonance imaging scan revealed soft tissue swelling, right nodularity and irregular nasal septal mucosal thinning, sinus mucosal thickening, and enhancing right jugular lymph nodes. Computed tomography of the chest yielded unremarkable results. The patient used smokeless chewing tobacco and electronic cigarettes but had an otherwise unremarkable exposure history.
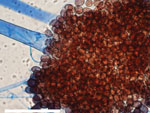
The patient underwent nasal endoscopy and debridement (Figure 1, panel B). Hematoxylin and eosin–stained sections from a biopsy of the right nasal septum revealed necrotic tissue with numerous hyaline fungal elements with a wide. ribbon-like appearance, further highlighted by Gomori methenamine-silver staining (Figure 1, panels C–F). Technicians isolated coagulase-negative Staphylococcus and Enterococcus faecium from bacterial cultures but considered those elements contaminants. We obtained 2 isolates from a fungal culture, identifying 1 microscopically, on lactophenol cotton blue stain, as a Curvularia species. Further testing by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Vitek MS V3.2; bioMérieux, https://www.biomerieux.com) revealed the isolate to be Curvularia lunata. We determined the other isolate to be Choanephora infundibulifera by phenotypic characterization (Figure 2) and BLAST searches (https://blast.ncbi.nlm.nih.gov/) using sequences of the nuclear ribosomal internal transcribed spacer region (GenBank accession no. OR643928) and the D1 and D2 domains of the 28S rRNA gene (GenBank accession no. OR643927). BLAST search results matched with reference strains as follows: internal transcribed spacer region, C. infundibulifera CBS 153.51 99.46%, C. infundibulifera KUS-F27535 99.49%; D1 and D2 domains of the 28S rRNA gene, C. infundibulifera CBS 153.51 100%, C. infundibulifera KUS-F27535 100% (1–3). We determined MICs for amphotericin B (≤0.3 μg/mL), micafungin (>8 μg/mL), voriconazole (>16 μg/mL), posaconazole (1 μg/mL), and isavuconazole (>16 μg/mL) per the Clinical and Laboratory Standards Institute’s broth microdilution method (4).
We initiated liposomal amphotericin B (5 mg/kg/d) as treatment and continued posaconazole. We directed the patient to receive 4 additional nasal and sinus debridements over 2 weeks, observing fungal elements in biopsies obtained from the first 3 operations. Cultures from all biopsies, however, were sterile. We transitioned the patient to amphotericin B (3×/wk) after 3 weeks and discontinued posaconazole 1 month thereafter. The patient’s nasal pain and tenderness progressively improved. Otolaryngology evaluation 4 weeks after the onset of infection was unremarkable. Unfortunately, the patient died of refractory leukemia 4 months after the diagnosis of his fungal infection (14 months after leukemia diagnosis).
The Mucorales group consists of over 260 species in 55 genera that are ubiquitous in wet, organic environments. Approximately 40 species are clinically significant, causing invasive infection (mucormycosis) chiefly in persons with diabetes and immunocompromising conditions (1). The genus Choanephora (family Choanephoraceae) contains 2 species, C. infundibulifera and C. cucurbitarum (5). These species are saprophytes or parasites of plants that can promote spoilage and disease (6). C. cucurbitarum, the more commonly recognized species, causes wet blight, flower rot blight, and leaf blight, chiefly on summer squash and other cucurbits (7).
First described by Currey in 1873, C. infundibulifera infrequently causes plant disease but has been implicated in twig and leaf rot and blossom blight (8–11). On potato-carrot or potato dextrose agar, colonies grow rapidly at 25°C with abundant white, pale-yellow, or brown mycelia and sporangiophores, with sporangia arising from substrate mycelium or nonseptate, unbranched, hyaline aerial hyphae (11). Definitive identification is based on morphology and sequencing of the nuclear ribosomal internal transcribed spacer region and the D1 and D2 domains of the 28S rRNA gene.
In the patient we describe, Curvularia species was among those isolated from the initial nasal biopsy, but histopathologic features observed in multiple biopsies over 2 weeks suggested that this was not the predominant pathogen. The fungal elements we observed in the infected tissue were consistently suggestive of an infection caused by a species in the order of Mucorales rather than a species of Curvularia, a dematiaceous mold that is typically pigmented, with septate and often acutely branched hyphae. Furthermore, the patient’s clinical course, with progressive tissue necrosis necessitating serial debridement to achieve a cure, was more consistent with the aggressive disease characteristic of a species in the order of Mucorales (12).
We could not determine a clear source of the patient’s fungal infection. We noted that he had limited exposure to the outdoors in the weeks before his infection and no close contact with plants or soil. We did not obtain hospital and domiciliary environmental samples; however, we did determine that no additional cases of infection caused by Choanephora or Curvularia species were reported in the hospital proximate to the patient’s illness.
The optimal treatment for infections caused by Choanephora species is unknown. The minimal inhibitory concentration correlation with treatment response in vivo is unknown, but the in vitro antifungal minimal inhibitory concentrations against this isolate suggest amphotericin B might have greater activity than posaconazole and isavuconazole, which are used to treat mucormycosis caused by other species. Consistent with the antifungal susceptibility results, our patient’s infection developed while he was receiving secondary prophylaxis with posaconazole. Treatment with liposomal amphotericin, initially in combination with posaconazole and with adjunctive surgical debridement, led to clinical and microbiological resolution of his infection despite ongoing cancer therapy and neutropenia. This report of human nasal infection caused by a species of Choanephora serves as a reminder that emerging fungal pathogens continue to pose clinical challenges, especially in severely immunocompromised patients.
Acknowledgments
We thank the staff of the St. Jude Children’s Research Hospital Biomedical Library for their research assistance.
This work was supported by a National Cancer Institute Comprehensive Cancer Center Support Grant (P30CA021765) and the American Lebanese Syrian Associated Charities.
Ms. Max is an advanced practice provider in the Department of Infectious Diseases, St. Jude Children’s Research Hospital, Memphis, Tennessee. Her research interest is infections in immunocompromised hosts.
This work was reviewed by the St. Jude Children’s Research Hospital Institutional Review Board.
References
- Walther G, Wagner L, Kurzai O. Updates on the taxonomy of Mucorales with an emphasis on clinically important taxa. J Fungi (Basel). 2019;5:106. DOIPubMedGoogle Scholar
- Lee SH, Nguyen TTT, Lee HB. Isolation and characterization of two rare Mucoralean species with specific habitats. Mycobiology. 2018;46:205–14. DOIPubMedGoogle Scholar
- Yin H, Tian M, Peng Y, Qin N, Lü H, Ren L, et al. First report on Choanephora cucurbitarum causing Choanephora rot in Chenopodium plants and its sensitivity to fungicide. J Fungi (Basel). 2023;9:881. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd edition (M38). Malvern (PA): Clinical and Laboratory Standards Institute; 2017 [cited 2023 Feb 27]. https://clsi.org/standards/products/microbiology/documents/m38
- Kirk PM. A monograph of the Choanephoraceae. Kew (UK): Commonwealth Agricultural Bureaux; 1984.
- Hoffmann K, Pawłowska J, Walther G, Wrzosek M, de Hoog GS, Benny GL, et al. The family structure of the Mucorales: a synoptic revision based on comprehensive multigene-genealogies. Persoonia. 2013;30:57–76. DOIPubMedGoogle Scholar
- Schuh M, Grabowski M. Choanephora rot. St. Paul (MN): University of Minnesota Extension; 2022 [cited 2023 Feb 27]. https://extension.umn.edu/disease-management/choanephora-rot
- Subba Rao KV, Padgett GB, Berner DK, Berggren GT, Snow JP. Choanephora leaf blight of soybeans in Louisiana. Plant Dis. 1990;74:614. DOIGoogle Scholar
- Alfieri SA Jr, Langdon KR, Wehlburg C, Kimbrough JW. Index of plant diseases in Florida (revised). Bull 11. Tallahassee, FL: Florida Department of Agriculture, Division of Consumer Services; 1984 [cited 2023 Feb 27]. https://original-ufdc.uflib.ufl.edu/UF00002948/00001/1j
- United States Department of Agriculture. Index of plant diseases in the United States. Washington: The Department of Agriculture, Agricultural Research Service; 1960.
- Chang YC, Graf E, Green AM. Invasive Curvularia infection in pediatric patients with hematologic malignancy identified by fungal sequencing. J Pediatric Infect Dis Soc. 2019;8:87–91. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: May 13, 2024
Table of Contents – Volume 30, Number 6—June 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Elisabeth Adderson, Department of Infectious Diseases, St. Jude Children’s Research Hospital, Mailstop 320, 262 Danny Thomas Pl, Memphis, TN 38105, USA
Top