Volume 30, Number 6—June 2024
Dispatch
Zoonotic Ancylostoma ceylanicum Infection in Coyotes from Guanacaste Conservation Area, Costa Rica, 2021
Abstract
Ancylostoma ceylanicum is the second most common hookworm infecting humans in the Asia-Pacific region. Recent reports suggest presence of the parasite in the Americas. We report A. ceylanicum infections in coyotes from the Guanacaste Conservation Area, Costa Rica. Our findings call for active surveillance in humans and animals.
The zoonotic hookworm Ancylostoma ceylanicum infects canids, felids, and humans in tropical regions of the world (1,2). Heavy infections may cause iron-deficiency anemia and bloody diarrhea as a result of ≈0.03 mL/worm/day blood loss caused by repeated blood meals taken from the intestines of infected persons by the parasites (2,3). Across the Asia-Pacific region, domestic and wild animals and humans, especially children and women of childbearing age, are at high risk for illness from infection with the parasite (2). The pooled proportion of A. ceylanicum hookworm–infected persons within the Asia Pacific is 12%, making it the second most common hookworm infecting humans in that region (4). However, the extent of its geographic distribution in the Americas and the range of animals this hookworm can infect are unknown.
In studies dating to 1922, adult hookworms, later identified as A. ceylanicum, were recovered from human cadavers from Brazil and, in 1976, from animals necropsied in Suriname, suggesting that the parasite might have been present in the Americas more than a century ago (2). However, except for those cases, research on A. ceylanicum hookworms in the Americas has remained neglected for a long time; that prolonged absence in research might be attributable to use of traditional microscopy techniques for hookworm diagnosis in the region (2). Those techniques are unable to differentiate hookworms at the species level, thereby limiting more detailed information on their distribution. More recently, applying molecular techniques has increased identification of regions endemic for A. ceylanicum hookworms (2). For example, in 1 recent study, samples from patients in Ecuador initially screened for hookworms in 2000 were reexamined, and A. ceylanicum hookworms were molecularly identified (5). In addition, A. ceylanicum hookworms have been reported in France in a migrant from Colombia and a traveler returning from French Guiana (6); in Germany, the parasite was reported in a child from Colombia (7). Furthermore, in 2022, endemicity of A. ceylanicum hookworms was molecularly confirmed in dogs in Grenada, West Indies (8). Despite the discovery of A. ceylanicum hookworms in wildlife elsewhere, initially in a civet cat native to Sri Lanka and subsequently in Asia in golden cats and leopards and in dingoes in Australia (9), no studies have reported infections in wildlife in the Americas.
This project was approved by the National System of Conserved Areas of Costa Rica (R-SINAC-ACG-003-2021). Costa Rica is in Central America within the equatorial tropical region. Among its 5 million residents, 766,000 are school-age children. Coyotes (Canis latrans) live around national parks and are widely distributed across the plains, mountains, forests, and tropics. To investigate the prevalence of hookworm and threadworm infections among coyotes, during May–October 2021, we collected and tested coyote scat from the Central Conservation Area in central and the Guanacaste Conservation Area in northwestern Costa Rica (10). To confirm coyotes were the origin of the collected fecal samples, we cross-referenced data from a separate study conducted on the same samples that employed restriction fragment-length polymorphism–based methods and metabarcoding analyses of the vertebrate 12S ribosomal RNA gene (11).
We extracted genomic DNA from fecal samples (n = 111) using the QIAamp PowerFecal Pro DNA Kit (QIAGEN, https://www.qiagen.com) according to manufacturer instructions and added an extra initial bead-beating step. We performed 2 TaqMan (Thermo Fisher Scientific; https://www.thermofisher.com) probe–based real-time quantitative PCRs in duplicate to detect all present canine hookworm species (A. ceylanicum, A. caninum, A. braziliense, and Uncinaria stenocephala) and Strongyloides spp. threadworms, as described elsewhere (10,12). We subjected samples positive for A. ceylanicum to molecular characterization of a partial region of the cox1 gene (13). We downloaded nucleotide cox1 sequences from dogs, humans, and cats from GenBank to assess the phylogenetic relationship among Ancylostoma spp. parasites and hosts.
We used MEGA11 (https://www.megasoftware.net) for aligning sequences and determining the best-fit substitution model. We conducted phylogenetic analyses using MrBayes version 3.2.7a (https://nbisweden.github.io/MrBayes/download.html) for Bayesian and RAxML version 8.2.12 (https://github.com/stamatak/standard-RAxML) for maximum-likelihood inference. We annotated the resulting phylogenic tree using TreeViewer version 1.17.6 (https://treeviewer.org) and constructed a haplotype network using PopArt (https://github.com/jessicawleigh/popart).
Of the 111 samples collected, 103 had sufficient feces for successfully extracting DNA, determined by amplifying a housekeeping gene of canids as DNA extraction control. Of those 103 samples, 59 (57.3%, 95% CI 46.6%–62.2%) harbored >1 zoonotic hookworms; A. caninum accounted for most infections (53.4%; 95% CI 43.3%–63.3%), followed by U. stenocephala (4.7%, 95% CI 1.5%–10.7%), and A. ceylanicum (2.8%, 95% CI 0.56%–8.05%). Strongyloides spp. threadworms were found in 3.8% (95% CI 1.04%–9.3%) of coyotes.
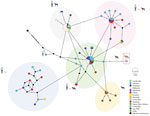
We obtained a 289-bp amplicon showing 99.6%–100% nucleotide identity with A. ceylanicum sequences from 2 positive A. ceylanicum samples (GenBank accession numbers OR801542 and OR801543). We determined phylogeny from 40 sequences that included A. ceylanicum sequences from dogs, cats, and humans, and U. stenocephala and A. caninum sequences from dogs. Phylogenetic analyses enabled separation of A. ceylanicum nucleotide sequences from coyote samples from Costa Rica from A. caninum and U. stenocephala sequences (Figure 1). Upon examining the median joining network of global A. ceylanicum sequences, we identified 5 distinct clusters, 4 of which were shared between domestic animals and humans and only 1 with dogs. We observed no clear population structure among countries represented by an admixture of haplotypes. A. ceylanicum haplotype from coyotes was unique but separated by only a few mutational steps from those from dogs and humans, and from zoonotic clusters (Figure 2).
We report zoonotic hookworm infections in coyotes from Costa Rica. Our data provide further evidence of the endemicity and local transmission of A. ceylanicum hookworm in the Americas and show that coyotes might play a role in transmitting this parasite to other animals. Even though coyotes are widely distributed across North and Central America and are established reservoirs of other zoonotic hookworms, such as A. caninum and U. stenocephala (14), their role as hosts of A. ceylanicum hookworm remains unexplored. Furthermore, detection of A. ceylanicum hookworms in wildlife poses questions about the parasite’s possible role as a disease agent in endangered animals, such as jaguars, giant anteaters, and Baird’s tapir, that live in sympatry in the Guanacaste Conservation Area (15).
Costa Rica has effectively controlled soil-transmitted helminths in humans, and residents no longer require preventive chemotherapy. However, the Pan American Health Organization still recommends continuous monitoring and evaluation to maintain control of soil-transmitted helminths (15). Although eliminating the need for preventive chemotherapy constituted a great milestone, doing so leaves untreated persons vulnerable to infection with zoonotic helminths, including A. ceylanicum. We therefore advocate for increased surveillance of A. ceylanicum hookworm in domestic animals, wildlife, and humans in the Americas to monitor and prevent transmission of zoonotic hookworms. Expanded surveillance is crucial considering active transmission of A. ceylanicum hookworm in humans has been reported in Ecuador, another country listed by the Pan American Health Organization as no longer requiring preventive chemotherapy (5,15). In-depth population genetics studies are needed to assess whether increases in the reports of A. ceylanicum hookworms in the Americas are attributable to recent human and animal migration from areas where the parasite is highly endemic. More robust epidemiologic data on A. ceylanicum infection in domestic animals and humans would help public health agencies assess the extent of local transmission in Costa Rica and other regions of the Americas and the role of animals in transmitting the parasite to humans.
Ms. Zendejas-Heredia is a PhD candidate at the University of Melbourne, Australia. Her research focuses on understanding the transmission dynamics of zoonotic soil-transmitted helminths.
Acknowledgment
We acknowledge Kevin Lloyd, Víctor Montalvo, Carolina Sáenz, Juan Carlos Cruz, and park rangers from the Central Conservation Area and Guanacaste Conservation Area for their collaboration in collecting fecal samples of coyotes.
References
- Smout FA, Skerratt LF, Butler JRA, Johnson CN, Congdon BC, Thompson RCA. The hookworm Ancylostoma ceylanicum: An emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health. 2017;3:66–9. DOIPubMedGoogle Scholar
- Traub RJ, Zendejas-Heredia PA, Massetti L, Colella V. Zoonotic hookworms of dogs and cats - lessons from the past to inform current knowledge and future directions of research. Int J Parasitol. 2021;51:1233–41. DOIPubMedGoogle Scholar
- Areekul S, Saenghirun C, Ukoskit K. Studies on the pathogenicity of Ancylostoma ceylanicum. I. Blood loss in experimental dogs. Southeast Asian J Trop Med Public Health. 1975;6:235–40.PubMedGoogle Scholar
- Clements ACA, Addis Alene K. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:e72–9. DOIPubMedGoogle Scholar
- Sears WJ, Cardenas J, Kubofcik J, Nutman TB, Cooper PJ. Zoonotic Ancylostoma ceylanicum hookworm infections, Ecuador. Emerg Infect Dis. 2022;28:1867–9. DOIPubMedGoogle Scholar
- Gerber V, Le Govic Y, Ramade C, Chemla C, Hamane S, Desoubeaux G, et al. Ancylostoma ceylanicum as the second most frequent hookworm species isolated in France in travellers returning from tropical areas. J Travel Med. 2021;28:28. DOIPubMedGoogle Scholar
- Poppert S, Heideking M, Agostini H, Fritzenwanker M, Wüppenhorst N, Muntau B, et al. Diffuse unilateral subacute neuroretinitis caused by Ancylostoma hookworm. Emerg Infect Dis. 2017;23:343–4. DOIPubMedGoogle Scholar
- Zendejas-Heredia PA, Colella V, Macpherson MLA, Sylvester W, Gasser RB, Macpherson CNL, et al. Ancylostoma ceylanicum hookworms in dogs, Grenada, West Indies. Emerg Infect Dis. 2022;28:1870–2. DOIPubMedGoogle Scholar
- Smout FA, Thompson RCA, Skerratt LF. First report of Ancylostoma ceylanicum in wild canids. Int J Parasitol Parasites Wildl. 2013;2:173–7. DOIPubMedGoogle Scholar
- Massetti L, Colella V, Zendejas PA, Ng-Nguyen D, Harriott L, Marwedel L, et al. High-throughput multiplex qPCRs for the surveillance of zoonotic species of canine hookworms. PLoS Negl Trop Dis. 2020;14:
e0008392 . DOIPubMedGoogle Scholar - Alfaro-Segura P, Robleto-Quesada J, Montenegro-Hidalgo VM, Molina-Mora JA, Baneth G, Verocai GG, et al. Elucidating Spirocerca lupi spread in the Americas by using phylogenetic and phylogeographic analyses. Front Parasitol. 2023;2:
1249593 . DOIGoogle Scholar - Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103:342–6. DOIPubMedGoogle Scholar
- Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis. 2014;20:976–82. DOIPubMedGoogle Scholar
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177–94. DOIPubMedGoogle Scholar
- Gyorkos T, Nicholls R, Montresor A, Luciañez A, Casapia M, St-Denis K, et al. Eliminating morbidity caused by neglected tropical diseases by 2030. Rev Panam Salud Publica. 2023;47:
e16 . DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: May 14, 2024
Table of Contents – Volume 30, Number 6—June 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Vito Colella, University of Melbourne, Melbourne Veterinary School, Parkville, VIC 3052, Australia
Top