Volume 30, Number 7—July 2024
Dispatch
Molecular Confirmation of Anopheles stephensi Mosquitoes in the Al Hudaydah Governorate, Yemen, 2021 and 2022
Cite This Article
Citation for Media
Abstract
We detected malaria vector Anopheles stephensi mosquitoes in the Al Hudaydah governorate in Yemen by using DNA sequencing. We report 2 cytochrome c oxidase subunit I haplotypes, 1 previously found in Ethiopia, Somalia, Djibouti, and Yemen. These findings provide insight into invasive An. stephensi mosquitoes in Yemen and their connection to East Africa.
Malaria remains a major threat to global health with ≈247 million cases reported in 2021 (1). An invasive malaria vector, Anopheles stephensi mosquito, has emerged in Africa; the first detection was in Djibouti in 2012 and was followed by detections in Ethiopia, Somalia, Sudan, Nigeria, Ghana, Kenya, and Eritrea (2) (Appendix, https://wwwnc.cdc.gov/EID/article/30/7/24-0331-App1.pdf). With growing evidence of An. stephensi mosquito resistance to multiple classes of insecticides (3,4), its association with a recent malaria outbreak (5), and genomic evidence that outbreak sites may also be central locations for An. stephensi mosquito travel to new areas (6), concerns are growing about the status and spread of this mosquito species in the Mediterranean region.
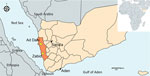
In the Arabian Peninsula, the geographic distribution of An. stephensi mosquitoes is unclear. Previous field investigations and predictive modeling indicate native populations exist in the northeastern coastal region along the Persian Gulf and inland in countries including Saudi Arabia (7,8). The status of An. stephensi mosquitoes is important in Yemen, where an increase in malaria cases was reported in the city of Aden beginning in 2017, although a link to An. stephensi mosquitoes has not been investigated (9). The first report of An. stephensi mosquitoes in Yemen occurred in Aden in 2021 and was confirmed with molecular analysis in 2023 (2,10). Recent retrieval of an unpublished entomological survey report indicated An. stephensi mosquitoes were present within the Al Zuhra district in the Al-Hudaydah governorate in 2000 (World Health Organization Yemen, unpub. data). No documentation of An. stephensi mosquitoes before 2000 or in later entomological surveys was found until the recent detection in 2021. Little is known about the distribution and characteristics of this vector in western governorates where the highest prevalence of malaria in Yemen is documented (9). An. stephensi mosquitoes were reported for the first time in the Ad Dahi district in December 2021 and in the Zabid district in March 2022, both within the Al Hudaydah governorate (2). An. stephensi mosquitoes have recently been found in multiple suburban areas in the Tehama coastal plain region (11). In this study, we characterize the genetic diversity of An. stephensi mosquitoes found during vector surveillance in the Al Hudaydah governorate.
We analyzed immature mosquitoes collected from 2 semiurban locations, Ad Dahi and Zabid districts (Figure 1; Appendix). We collected the Ad Dahi district specimens over the course of a single day in December 2021 while conducting Aedes surveillance during a dengue fever outbreak. The Zabid district specimens were collected during monthlong Anopheles surveillance in March 2022. Potential breeding containers surveyed included open concrete ponds and cement water tanks near block factories, washing basins in mosques, and car washing sites. Mosquitos used in this study were collected as immature specimens by using the dipping method. We reared them to adults in field insectaries and then identified them by using updated morphological keys (12). The specimens we morphologically identified as An. stephensi mosquitoes were preserved with silica gel. We sent a subsample of specimens to Baylor University (Waco, Texas, USA) for molecular analysis.
For species identification, we analyzed 2 loci, cytochrome c oxidase subunit 1 (COI) and internal transcribed spacer 2 (ITS2), by using previously described protocols (13). We used an An. stephensi–specific endpoint PCR as previously described (14). We conducted ITS2- and COI-targeted DNA sequencing, then used BLAST (https://blast.ncbi.nlm.nih.gov) and conducted phylogenetic analysis to determine species identification and evaluate the level of genetic diversity (Appendix).
Most of the Anopheles larvae were collected from cement water tanks found outside of homes, which were like other invasive settings found in eastern Ethiopia and Aden (4,10). We identified 41 mosquitoes morphologically as An. stephensi, 7 from Ad Dahi and 34 from Zabid City. Of the 41 specimens, all 7 Ad Dahi and 32 of the Zabid City specimens were confirmed to be An. stephensi mosquitoes by ITS2 endpoint assay and by COI and ITS2 targeted DNA sequence analysis (Appendix). We observed no discrepancy between the endpoint assay and sequencing assay for species identification. We did not report any discrepancies with the ITS2 endpoint assay results when compared with the DNA sequence results, but incorrect identification with the endpoint assay for other populations with unreported ITS2 variation is possible. Sequencing should accompany initial implementation of an endpoint assay in newly surveyed populations to evaluate both false negatives and false positives.
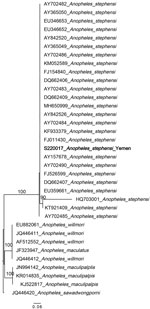
Two non–An. stephensi mosquitoes were recovered; 1 was identified as Anopheles culicifacies and 1 as Aedes aegypti on the basis of ITS2 and COI sequence BLAST analysis. This change from the initial morphological identification could be the result of sorting the Anopheles and Aedes mosquitoes from the same habitat or from the selection of specimens for molecular analysis in the laboratory. Phylogenetic analysis confirmed the species identification of the An. stephensi mosquito specimens (bootstrap = 100 for COI, bootstrap = 100 for ITS2) (Figures 2, 3).
ITS2 sequences were all identical for the An. stephensi mosquito specimens and BLAST analysis revealed a 100% sequence identification in GenBank. Sequence analysis of COI revealed 2 haplotypes. One haplotype (n=35) was previously reported throughout the Horn of Africa (HoA) (COIHap 3) with the highest frequency in northeastern Ethiopia, Somaliland, and Djibouti (15). This haplotype was also noted in a recent report on the detection of An. stephensi mosquitoes in Aden, Yemen (10). The second haplotype (n=4), differing by a single nucleotide from COIHap3, has not previously been reported (designated COIHapYem1).
These findings demonstrate the successful use of ongoing vector surveillance activities for the initial detection of An. stephensi mosquitoes. The detection of a common HoA COI haplotype raises questions about the relationship between the invasive An. stephensi mosquitoes in northern HoA and Yemen. It is possible they share a common origin or that movement of An. stephensi mosquitoes has occurred between the 2 regions. Sequencing of additional loci could assist with delineating their relationship. Our findings provide information about the relative timing of the Yemen introduction of An. stephensi mosquitoes relative to the HoA detection on the basis of several observations. Fewer haplotypes were observed in the Al Hudaydah region in comparison to northeastern Ethiopia, Somalia, and Djibouti, which may indicate a recent introduction into the Al Hudaydah region. COIHap3 has a limited geographic range in comparison to the HoA-wide Hap2, which suggests the haplotype from our study could be associated with a later introduction in HoA and potentially Yemen. As for the HoA specimens and the study in Aden, no Saudi Arabian haplotypes were detected. Unlike the HoA specimens, no South Asia haplotypes were detected. Further genomic analysis and extensive An. stephensi mosquito sampling in Yemen, Saudi Arabia, and other parts of the Arabian Peninsula are needed to evaluate the hypothesis of a recent introduction in Yemen relative to the HoA.
Our findings provide insight into the genetic diversity of An. stephensi mosquito populations in Al Hudaydah governorate, Yemen. The findings also provide support for the need of long-term entomological and epidemiologic surveillance of An. stephensi mosquitoes on malaria transmission in the region. With the detection of An. stephensi mosquitoes in Yemen, concerns remain related to the status of these mosquitoes in other areas of Yemen and their relationship with the HoA An. stephensi mosquitoes. Additional genomic analysis should be conducted to further examine the relative timing of the introduction of An. stephensi mosquitoes to Yemen.
Dr. Methaq is an epidemiologist and director of the National Malaria Control Program, Ministry of Health, Sana’a, Yemen. His interests include the species composition and bionomics of mosquito vectors in Yemen.
Acknowledgments
We would like to thank Dr. Ghasem Zamani for his contribution to the conceptualization of the project, technical support for implementation of surveillance, and review of the manuscript. We would also like to thank Yamaan Foundation for Health and Social Development for their support of this work.
This research was funded by a NIH Research Enhancement Award (1R15AI151766) awarded to Tamar E. Carter and Baylor University.
Author contributions: Conception and project design (T.E.C., M.A., and S.A.E.); specimen collection and organization (M.A., M.A.H., M.A.E., J.A.J., and A.S.); data generation (T.E.C., P.A., and J.S.); data analysis (T.E.C. and P.A.); first draft (T.E.C.). All authors read and approved the final version of this manuscript.
References
- World Health Organization. World malaria report 2023. [cited 2024 Feb 1]. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023
- World Health Organization. Malaria Threats Map. [cited 2021 Sep 1]. https://apps.who.int/malaria/maps/threats
- Yared S, Gebressielasie A, Damodaran L, Bonnell V, Lopez K, Janies D, et al. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar J. 2020;19:180. DOIPubMedGoogle Scholar
- Balkew M, Mumba P, Yohannes G, Abiy E, Getachew D, Yared S, et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018-2020. Malar J. 2021;20:263. DOIPubMedGoogle Scholar
- Emiru T, Getachew D, Murphy M, Sedda L, Ejigu LA, Bulto MG, et al. Evidence for a role of Anopheles stephensi in the spread of drug- and diagnosis-resistant malaria in Africa. Nat Med. 2023;29:3203–11. DOIPubMedGoogle Scholar
- Samake JN, Lavretsky P, Gunarathna I, Follis M, Brown JI, Ali S, et al. Population genomic analyses reveal population structure and major hubs of invasive Anopheles stephensi in the Horn of Africa. Mol Ecol. 2023;32:5695–708. DOIPubMedGoogle Scholar
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. DOIPubMedGoogle Scholar
- Munawar K, Saleh A, Afzal M, Qasim M, Khan KA, Zafar MI, et al. Molecular characterization and phylogenetic analysis of anopheline (Anophelinae: Culicidae) mosquitoes of the Oriental and Afrotropical Zoogeographic zones in Saudi Arabia. Acta Trop. 2020;207:
105494 . DOIPubMedGoogle Scholar - Al-Eryani SM, Irish SR, Carter TE, Lenhart A, Aljasari A, Montoya LF, et al. Public health impact of the spread of Anopheles stephensi in the WHO Eastern Mediterranean Region countries in Horn of Africa and Yemen: need for integrated vector surveillance and control. Malar J. 2023;22:187. DOIPubMedGoogle Scholar
- Allan R, Weetman D, Sauskojus H, Budge S, Hawail TB, Baheshm Y. Confirmation of the presence of Anopheles stephensi among internally displaced people’s camps and host communities in Aden city, Yemen. Malar J. 2023;22:1. DOIPubMedGoogle Scholar
- Entomological Report NMCP. National Malaria Control Programme MoPHaP. 2022.
- Coetzee M. Key to the females of afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J. 2020;19:70. DOIPubMedGoogle Scholar
- Waymire E, Samake JN, Gunarathna I, Carter TE. A decade of invasive Anopheles stephensi sequence-based identification: toward a global standard. Trends Parasitol. 2024;40:477–86. DOIPubMedGoogle Scholar
- Djadid ND, Gholizadeh S, Aghajari M, Zehi AH, Raeisi A, Zakeri S. Genetic analysis of rDNA-ITS2 and RAPD loci in field populations of the malaria vector, Anopheles stephensi (Diptera: Culicidae): implications for the control program in Iran. Acta Trop. 2006;97:65–74. DOIPubMedGoogle Scholar
- Carter TE, Yared S, Getachew D, Spear J, Choi SH, Samake JN, et al. Genetic diversity of Anopheles stephensi in Ethiopia provides insight into patterns of spread. Parasit Vectors. 2021;14:602. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: June 18, 2024
Table of Contents – Volume 30, Number 7—July 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Tamar E. Carter 101 Bagby Ave Rm A124, Waco, TX, USA, 76706
Top