Volume 30, Number 9—September 2024
Research
Lower Microscopy Sensitivity with Decreasing Malaria Prevalence in the Urban Amazon Region, Brazil, 2018–2021
Cite This Article
Citation for Media
Abstract
Malaria is increasingly diagnosed in urban centers across the Amazon Basin. In this study, we combined repeated prevalence surveys over a 4-year period of a household-based random sample of 2,774 persons with parasite genotyping to investigate the epidemiology of malaria in Mâncio Lima, the main urban transmission hotspot in Amazonian Brazil. We found that most malarial infections were asymptomatic and undetected by point-of-care microscopy. Our findings indicate that as malaria transmission decreases, the detection threshold of microscopy rises, resulting in more missed infections despite similar parasite densities estimated by molecular methods. We identified genetically highly diverse populations of Plasmodium vivax and P. falciparum in the region; occasional shared lineages between urban and rural residents suggest cross-boundary propagation. The prevalence of low-density and asymptomatic infections poses a significant challenge for routine surveillance and the effectiveness of malaria control and elimination strategies in urbanized areas with readily accessible laboratory facilities.
Despite recent progress toward elimination, persisting malaria transmission in the Americas continues to pose a risk for infection to 120 million persons (1). The Amazon Basin, spanning 9 countries of South America, accounts for ≈90% of the 600,000 laboratory-confirmed cases recorded annually on the continent (2). The actual malaria burden may be significantly underestimated because point-of-care microscopy is not sensitive enough to detect the parasites in all low-density infections, most of which are asymptomatic, in the Amazon Basin (3–5).
Traditionally considered a rural disease, malaria is now emerging in rapidly growing urban centers across the Amazon Basin (6–8). After decades of massive rural-to-urban migration, 75% of the population in the Brazilian Amazon now resides in settlements classified as urban according to municipality law (https://censo2022.ibge.gov.br/panorama). This classification is adopted by the Brazilian Institute of Geography and Statistics, the country’s census bureau. Urban health facilities, generally more accessible and better equipped than their rural counterparts, can provide prompt malaria diagnosis and treatment and prevent new infections (9). However, chronic asymptomatic and submicroscopic infections can escape detection and sustain transmission. The rapid, unplanned urbanization of this region may transform malaria into an urban disease in the Amazon Basin, with broad public health implications.
In this study, we combined population-based prevalence data and parasite genotyping to examine the epidemiology of residual malaria in the main urban transmission hotspot of Brazil. We tested the hypothesis that, once introduced into receptive urbanized spaces, malaria parasites spread unnoticed among asymptomatic carriers, potentially causing outbreaks more frequently than parasite lineages restricted to remote rural villages.
The Institutional Review Board of the Institute of Biomedical Sciences, University of São Paulo, and the National Committee on Ethics in Research of the Ministry of Health of Brazil approved this study protocol (CAAE no. 6467416.6.0000.5467). We obtained written informed consent and assent from all study participants or their parents or guardians.
Study Site
The municipality or county of Mâncio Lima (Figure 1) (7) had an annual parasite incidence (number of laboratory-confirmed malaria cases per 1,000 persons per year) estimated at 422.8, the highest in Brazil, at the study onset in 2018. Half of the municipality’s population lived in the town of Mâncio Lima (Appendix Figure 1), which accounted for 48% of the municipality’s malaria cases in 2018. Anopheles (Nyssorhynchus) darlingi, the primary malaria vector, thrives in fish farming tanks and ponds that were opened across the town starting in the early 2000s (10).
Household Panel Survey
We analyzed biological specimens and data from a household-based random sample comprising 20% of the residents in the town of Mâncio Lima (11). Our analysis included repeated measurements on a cohort of residents in the same households to characterize temporal changes in the outcomes of interest (12). Study waves took place in April‒May 2018 (wave 1), September‒October 2018 (wave 2), May‒June 2019 (wave 3), September‒October 2019 (wave 4), October‒November 2020 (wave 5), April‒May 2021 (wave 6), and October‒November 2021 (wave 7). Over the 4-year study period, the incidence of microscopy-confirmed malaria decreased markedly among urban residents (Figure 2).
We recorded sociodemographic and illness information and used a household-based wealth index as a proxy of socioeconomic status (Appendix). We collected finger-prick capillary blood samples from participants >3 months of age, regardless of any symptoms, for malaria diagnosis and parasite genotyping. We asked participants whether they had experienced any signs or symptoms that might have been caused by a malarial infection <7 days before the interview. We specifically asked those reporting any signs or symptoms whether they had fever, chills, or headache.
Laboratory Diagnosis of Malaria
We stained thick blood smears with Giemsa (Merck KGaA, https://www.emdgroup.com) and examined for malaria parasites under 1,000× magnification; a research microscopist who had >15 years of experience reviewed >200 fields. The protocols for slide staining and reading remained consistent throughout the study period. We also tested blood samples for malarial antigens, during waves 3 and 4, using the QuickProfile Pf/Pv (Lumiquick, https://lumiquick.com) rapid diagnostic test, and used them for DNA extraction followed by molecular diagnosis, during all study waves (Appendix).
We initially screened samples with a genus-specific PCR that targets a conserved sequence of the cytb gene of human-infecting malaria parasites (13) using a detection threshold of 0.2 amplicon copies/µL, equivalent to as few as 4 parasites/mL, assuming an average of 50 mitochondrial genome copies per blood-stage parasite. We further tested positive samples with separate species-specific quantitative TaqMan assays (ThermoFisher Scientific, https://www.thermofisher.com) that targeted mitochondrial genome sequences of P. vivax (84-bp domain of the cox1 gene) or P. falciparum (90-bp domain spanning the 3′ end of the cox1 gene and the nearby intergenic region) (14). Those techniques amplify circulating DNA from peripheral-blood parasites in addition to DNA released from dead parasites (15) sequestered in capillaries or accumulating in extravascular spaces of the bone marrow and spleen, providing an indirect estimate of the total parasite burden harbored by the host.
Parasite Sampling and Genotyping
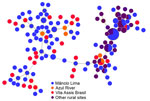
To map the spread of parasite lineages across the region, we genotyped isolates collected during the panel study waves (Mâncio Lima sample set) along with 3 additional sample sets (Table 1; Appendix Figure 2). The Azul River/Nova Cintra set comprised samples obtained in April 2016 during cross-sectional surveys in riverine villages along Azul River, 6–8 hours by motorboat from the municipality of Mâncio Lima, and Nova Cintra on the Juruá River, municipality of Rodrigues Alves, in addition to samples collected in July 2018 and July 2019 along Azul River (14). The Vila Assis Brasil set included samples collected during cross-sectional surveys in August–September 2018, March 2019, and September–October 2019 in a periurban village 15 km away from Mâncio Lima by paved road (16). The health facility sample set consisted of isolates from symptomatic patients with microscopy-confirmed malaria who attended clinics in the municipalities of Mâncio Lima, Cruzeiro do Sul, and Rodrigues Alves during April 2018‒April 2020; most of those samples were collected in 2019 (11). We inferred the likely sites of infection by considering patients’ places of residence and history of recent travel.
We typed 6 microsatellite loci for each species: MS2, MS5, MS6, MS7, MS9, and MS15 for P. vivax (17) and polyα, TAA81, TAA42, TA87, TA109, and TA60 for P. falciparum (18) (Appendix Table 1). We defined haplotypes as unique combinations of alleles at each locus, considering only the most abundant allele when >2 alleles were detected in the same sample (Appendix). We calculated the expected heterozygosity as an estimate of genetic diversity and the standardized index of association as a measure of multilocus linkage disequilibrium with LIAN 3.7 software as previously described (19) (Appendix). We used the goeBURST algorithm implemented in PHYLOViZ (https://www.phyloviz.net) to identify clusters of genetically related haplotypes (20) that might be informative about parasite migration patterns (Appendix Figure 3).
Statistical Analysis
We entered data using the REDCap system (https://projectredcap.org/software) and analyzed using Stata 15.1 software (StataCorp LLC, https://www.stata.com). We defined statistical significance at the 5% level for 2-tailed tests. We calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy, along with their 95% CIs, for microscopy and clinical signs and symptoms as predictors of infection detected by molecular methods (Appendix).
We used multivariable mixed-effects logistic regression models to identify correlates of malarial infection and disease while adjusting for potential confounders. We performed separate analyses for the following outcomes: TaqMan-confirmed P. vivax, regardless of any symptoms; TaqMan-confirmed P. falciparum infection, regardless of any symptoms; and clinical malaria (TaqMan-confirmed P. vivax or P. falciparum infection and reported fever, chills, or headache within the previous 7 days) (Appendix). We estimated odds ratios (ORs) with 95% CIs to quantify the influence of each predictor on the outcome. We also used logistic regression models (21) to describe the detectability of infection by microscopy as a continuous function of the number of species-specific amplicon copies per microliter measured by TaqMan.
Prevalence of Submicroscopic and Asymptomatic Infection
We analyzed data from 2,774 residents in Mâncio Lima, ranging in age from 4 months to 103 years and distributed into 879 households, who underwent malaria parasite screening during >1 study wave. The number of participants varied from 1,093 in wave 1 to 2,043 in wave 7 (Appendix Table 2); 529 (19.1%) persons were screened at all time points, whereas 402 (14.5%) participated in a single study wave. Out of 11,730 specimens screened by PCR, 11,717 (99.9%) were also tested by microscopy.
PCR followed by TaqMan detected ≈10 times more infections than microscopy: out of 11,730 samples screened, 467 (4.0%) were positive for Plasmodium vivax, 104 (0.9%) for P. falciparum, and 54 (0.5%) for both species. Microscopy revealed 42 (0.4%) samples positive for P. vivax, 18 (0.2%) for P. falciparum, and 1 (0.01%) for both species, among 11,717 microscopically examined samples; we identified no other Plasmodium species (Table 2). None of the 54 mixed-species infections detected by TaqMan had been correctly identified by microscopy. Conversely, the only mixed-species infection diagnosed by microscopy yielded a positive TaqMan result for P. vivax only (Appendix Table 3).
Compared with molecular methods, microscopy demonstrated a diagnostic sensitivity of 8.5% (95% CI 6.5%–11.1%), specificity of 99.9% (95% CI 99.9%–100.0%), PPV of 86.9% (95% CI 75.9%–93.3%), NPV of 95.1% (95% CI 95.0%–95.2%), and accuracy of 95.1% (95% CI 94.7%–95.5%) (Appendix Tables 3, 4). The proportion of molecularly diagnosed malarial infections that were asymptomatic varied from 73.8% in wave 2 to 96.8% in wave 6, with a higher proportion of asymptomatic infections during the latest study waves (Table 2). When compared with PCR followed by Taqman, the overall diagnostic sensitivity of reported fever, chills, or headache was only 16.0% (95% CI 13.2%–19.1%); specificity was 90.8% (95% CI 90.3%–91.4%), PPV 8.9% (95% CI 7.53%–10.6%), NPV 95.1% (95% CI 94.9%–95.2%), and accuracy 86.8% (95% CI 86.2%–87.5%). Overall, we observed a higher prevalence of infection with P. vivax and P. falciparum and of clinical malaria among adolescent and adults from the poorest households in the town (Appendix Tables 2,5), as indicated by adjusted multiple logistic regression analysis incorporating >11,000 observations.
Parasite Detectability over Time
The prevalence of malarial infection diagnosed by microscopy and molecular methods, either symptomatic or asymptomatic, significantly declined during the study period (Figure 2; Appendix Table 2). The later study waves exhibited an increased proportion of submicroscopic infections, which might be attributed to lower average parasite densities during periods of decreased transmission (21). However, TaqMan-quantified P. vivax densities remained consistent during 2018–2021 at an overall geometric mean of 19.0 amplicon copies/μL (Appendix Table 2). In contrast, P. falciparum densities (geometric mean 10.5 amplicon copies/μL) fluctuated more prominently, from 3.2 amplicon copies/μL in wave 6 to 86.3 amplicon copies/μL in wave 5, although the limited sample size prevented robust identification of temporal trends (Appendix Table 2).
The likelihood of detecting parasite by microscopy as a function of TaqMan-measured parasite density diminished with time. In 2018, we anticipated to identify 50% of P. vivax infections by microscopy at a density of 2,088 (95% CI 734–14,572) amplicon copies/μL or ≈42 parasites/μL (Figure 3, panel A). Nevertheless, most P. vivax infections above this threshold were undetected by microscopy in subsequent years, consistent with a decline in microscopy sensitivity. At a given TaqMan-measured average parasite density, the diagnostic sensitivity of peripheral-blood microscopy decreased during periods of lowest malaria prevalence. Despite the considerably smaller sample size, we noted a similar temporal trend in the microscopic diagnosis of P. falciparum infections (Figure 3, panel B). Those findings suggest that a higher proportion of infections are overlooked by peripheral-blood microscopy as malaria prevalence declines.
Parasite Lineages over Space and Time
During 2018–2021, we genotyped 122 P. vivax and 92 P. falciparum isolates collected in the town of Mâncio Lima, along with 142 P. vivax and 70 P. falciparum isolates from surrounding rural areas (Table 1; Appendix Figure 2). The parasites exhibited a high genetic diversity (average heterozygosity >0.8 for both species) that varied little over time in Mâncio Lima (Appendix Table 2). Both P. vivax and P. falciparum populations displayed substantial linkage disequilibrium (Appendix), consistent with the circulation of near-clonal parasite lineages across the region (22). Of interest, minimal spanning trees showed no clear clustering based on infection sites in the Juruá Valley for P. vivax (Figure 4) and P. falciparum (Figure 5). Instead, parasite haplotypes from the town of Mâncio Lima and the rural surroundings were evenly distributed in the trees. We observed 1 instance for P. vivax and 6 instances for P. falciparum of haplotype sharing among parasites from the town and rural areas, indicating substantial connectivity between urban and rural populations.
We observed some evidence of temporal clustering of parasite lineages (Appendix). Parasites from both species circulating in the town of Mâncio Lima during 2019 formed clusters (Appendix Figure 4) that were enriched in lineages from symptomatic and patent infections (Appendix Figure 5). Similarly, clusters of patent and symptomatic P. vivax and P. falciparum infections were also observed in the analysis of the complete Juruá Valley dataset, combining data from urban and rural sites (Appendix Figure 6).
In this extensive population-based study spanning 4 years, we found increasing proportions of asymptomatic and submicroscopic infections as the overall number of urban malaria cases decreased in the main transmission hotspot of Brazil. Of notable practical importance, the utility of microscopic diagnosis for detecting parasitemia diminished as malaria transmission decreased, which carries medical and public health implications. This challenge is not unique to our study site; microscopy has been demonstrated to overlook a higher proportion of P. falciparum infections in the areas of lowest malaria prevalence across sub-Saharan Africa (23). Molecular diagnosis may be necessary for identifying low-density malarial infections in low transmission settings, where microscopy-based parasite prevalence is <10% (24). However, new field-deployable tests for parasite DNA detection should undergo validation before widespread implementation in peripheral laboratories in the Amazon Basin (5). Furthermore, positive PCR results should be interpreted as indicative of either current or recent infection (15).
Few studies have identified changes in microscopy sensitivity with decreasing malaria transmission by repeatedly sampling the same population (16,25). Here, we provide evidence of rising microscopy detection thresholds, showing that a higher proportion of P. vivax and P. falciparum infections were missed at comparable parasite densities as malaria incidence diminished over time. Of note, molecular methods can detect circulating DNA released by dead trophozoites and schizonts that may have been concealed in capillaries and venules or extravascular territories (15). We speculate that, at lower transmission, fewer parasites accumulating in deep-organ blood vessels and extravascular spaces tend to circulate in the peripheral blood, where they can be identified by microscopy, perhaps indicative of parasites’ adaptation to seasonal variation in vector abundance (26) or enhanced control measures (27). Temporal changes in P. falciparum sequestration patterns, particularly in regions with seasonal malaria transmission (26), can theoretically influence parasite detectability in the peripheral blood. However, additional longitudinal studies are required to validate this hypothesis.
Our findings demonstrate that parasite lineages spread across rural‒urban boundaries, suggesting that rural areas with limited diagnosis and surveillance may serve as important sources for malaria reintroduction. Rural‒urban mobility introduces malaria parasites into receptive, more densely populated urbanized spaces (28). The most mobile residents of Mâncio Lima are men 16‒60 years of age who lack formal employment in the town and are often engaged in subsistence or commercial farming in periurban settlements (29). We found that closely related lineages of P. vivax and P. falciparum are shared among urban and rural residents, indicating the free circulation of malaria parasites across the rural‒urban boundaries in the Juruá Valley. Efficient parasite spreaders, such as forestgoers in Southeast Asia villages (30), can be selectively targeted for more intensive and effective malaria control interventions that cannot be readily delivered to the entire community, in this and similar endemic settings across the Amazon.
Brazil has an ambitious plan to achieve zero malaria by reducing cases from 150,000 in 2022 to <68,000 by 2025, eliminating P. falciparum malaria and associated deaths by 2030, eliminating cases and deaths by any species by 2035, and preventing malaria reintroduction from 2035 onward (31). However, elimination plans can be undermined by the establishment of malaria transmission in highly connected urbanized spaces that serve as both sinks and sources of parasite lineages. The large urban and periurban malaria outbreaks recorded in the Juruá Valley since the mid-2000s, when the region became the main transmission hotspot of Brazil, have been linked to the opening of fishponds for commercial aquaculture (6,7,32). By 2015, the Juruá Valley contributed 18% of the country’s malaria burden (33). Although most infections diagnosed during our study were asymptomatic, parasite carriers are exposed to recurrent episodes of parasitemia, which lead to chronic anemia and other possible clinical and immunologic consequences (34). Of importance, the hidden parasite reservoir missed by routine surveillance substantially contributes to human-to-mosquito parasite transmission in similar low-endemicity settings (4,21,35,36).
The country’s strategy for malaria surveillance and control comprises several actions: to provide prompt microscopy-based diagnosis and treatment after detection of isolated cases or clusters of cases; to detect additional cases, once an index case has been diagnosed; and to implement vector control measures in the vicinity of passively detected cases (37). Active surveillance around cases has gradually been implemented in Brazil since reactive case detection was demonstrated to be feasible and effective for malaria control in the Amazon (38), although molecular diagnosis is rarely used with this purpose.
The role of long-lasting insecticide-treated bed nets (LLINs) and indoor residual spraying in malaria control in Mâncio Lima is uncertain. Only 46% of the study participants reported using LLINs the previous night; <15% had their houses sprayed within the previous 12 months (Appendix Table 5). Larval source management is currently limited to periodically removing vegetation from the margins of fish farming tanks and emptying those no longer used for aquaculture. Alternatively, the periodic application of environmentally safe biolarvicides can drastically reduce anopheline larval density in fish tanks (39), but its feasibility for large-scale use remains to be determined.
The primary strength of this study lies in its household panel design (12), which enabled us to examine temporal trends of malarial infection and disease at the community level and to identify changes in the sensitivity of diagnostic microscopy over 4 years. The first limitation of this study is that only 61 microscopy-positive infections and 100 clinical malaria episodes were diagnosed during 7 study waves, despite screening a large population, as expected under low transmission. Second, we defined clinical malaria on the basis of perceived signs and symptoms, rather than fever measured at the time of interviews. We argue that perceived symptoms, although not highly specific and prone to recall bias, are the key drivers of healthcare seeking and define which infections will be identified by passive surveillance and eventually treated. Third, microsatellite genotyping provides a relatively limited view of genetic variation and connectivity among local parasites, compared with targeted amplicon sequencing (40) or whole-genome analysis (41). However, our results are broadly consistent with genome-wide analyses that showed recent ancestry sharing between urban and rural near-clonal lineages of P. vivax across Juruá Valley (22).
In conclusion, our research illustrates how parasite lineages can spread across rural‒urban boundaries and cause predominantly asymptomatic infections that are increasingly missed by microscopy as the overall malaria incidence drops. The presence of an undetected urban malaria reservoir may require more effort toward regional control and elimination strategies.
Dr. Rodrigues is a staff researcher at the Brazilian Center for Research in Energy and Materials in Campinas, Brazil. Her research interests have recently shifted from malaria diagnosis and molecular epidemiology to the development of robust and efficient microbial cell factories.
Author contributions: P.T.R., I.C.J., J.M.V., M.C.C. and M.U.F. contributed to the study design and implementation; P.T.R., I.C.J., W.A.L., J.T., R.M.C., A.R.J.F., P.S.F. C.E.C., and M.U.F contributed to field data collection; P.T.R., W.A.L., F.D.E., J.T., and P.R.C. contributed to methods and laboratory analyses; I.C.J, R.M.C., A.R.J.F., M.C.C., and M.U.F. contributed to data management, formal analysis, and visualization; J.M.V. and M.U.F. contributed to project management. All authors contributed to the writing and reviewed and approved the final manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
We thank the 2,774 participants in the Mâncio Lima Urban Cohort Study. We thank Ajucilene (Joice) G. Mota, Francisco Melo, and their team at the Health Secretary of Mâncio Lima for their overall logistic support; Juliana C. Belizário, Bárbara Prado C. Silva, Vanessa C. Nicolete, Amanda O. S. Fernandes, and Isabel Giacomini for fieldwork; Jaques N. de Carvalho for laboratory support, and Maria José Menezes for administrative support.
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (2016/18740‐9 to M.U.F.), the National Institutes of Health (grant U19 AI089681 to J.M.V.), and the Fundação para a Ciência e Tecnologia, Portugal (institutional GHTM project, UID/04413/2020). FAPESP provided scholarships to P.T.R., I.C.J., W.A.L., F.D.E., and P.S.F; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, provided scholarships to R.M.C. and M.U.F.; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior provided a scholarship to W.A.L. The funders had no role in study design, data collection and interpretation, or in the decision to submit the work for publication.
References
- World Health Organization. World malaria report 2022. Geneva: The Organization; 2022.
- Ferreira MU, Castro MC. Malaria situation in Latin America and the Caribbean: residual and resurgent transmission and challenges for control and elimination. Methods Mol Biol. 2019;2013:57–70. DOIPubMedGoogle Scholar
- Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–40. DOIPubMedGoogle Scholar
- Ferreira MU, Corder RM, Johansen IC, Kattenberg JH, Moreno M, Rosas-Aguirre A, et al. Relative contribution of low-density and asymptomatic infections to Plasmodium vivax transmission in the Amazon: pooled analysis of individual participant data from population-based cross-sectional surveys. Lancet Reg Health Am. 2022;9:
100169 . DOIPubMedGoogle Scholar - Torres K, Ferreira MU, Castro MC, Escalante AA, Conn JE, Villasis E, et al. Malaria Resilience in South America: Epidemiology, Vector Biology, and Immunology Insights from the Amazonian International Center of Excellence in Malaria Research Network in Peru and Brazil. Am J Trop Med Hyg. 2022;107(Suppl):168–81. DOIPubMedGoogle Scholar
- Costa KM, de Almeida WA, Magalhães IB, Montoya R, Moura MS, de Lacerda MV. [Malaria in Cruzeiro do Sul (Western Brazilian Amazon): analysis of the historical series from 1998 to 2008] [in Portuguese]. Rev Panam Salud Publica. 2010;28:353–60. DOIPubMedGoogle Scholar
- Olson SH, Gangnon R, Silveira GA, Patz JA. Deforestation and malaria in Mâncio Lima County, Brazil. Emerg Infect Dis. 2010;16:1108–15. DOIPubMedGoogle Scholar
- Salla LC, Rodrigues PT, Corder RM, Johansen IC, Ladeia-Andrade S, Ferreira MU. Molecular evidence of sustained urban malaria transmission in Amazonian Brazil, 2014-2015. Epidemiol Infect. 2020;148:
e47 . DOIPubMedGoogle Scholar - Wilson ML, Krogstad DJ, Arinaitwe E, Arevalo-Herrera M, Chery L, Ferreira MU, et al. Urban malaria: understanding its epidemiology, ecology, and transmission across seven diverse ICEMR network sites. Am J Trop Med Hyg. 2015;93(Suppl):110–23. DOIPubMedGoogle Scholar
- dos Reis IC, Codeço CT, Degener CM, Keppeler EC, Muniz MM, de Oliveira FG, et al. Contribution of fish farming ponds to the production of immature Anopheles spp. in a malaria-endemic Amazonian town. Malar J. 2015;14:452. DOIPubMedGoogle Scholar
- Johansen IC, Rodrigues PT, Tonini J, Vinetz J, Castro MC, Ferreira MU. Cohort profile: the Mâncio Lima cohort study of urban malaria in Amazonian Brazil. BMJ Open. 2021;11:
e048073 . DOIPubMedGoogle Scholar - Lipps O. Panel surveys: advantages and disadvantages compared with repeated cross-sectional surveys. FORS guide no. 14, version 1.0. Lausanne: Swiss Centre of Expertise in the Social Sciences FORS; 2021.
- Putaporntip C, Buppan P, Jongwutiwes S. Improved performance with saliva and urine as alternative DNA sources for malaria diagnosis by mitochondrial DNA-based PCR assays. Clin Microbiol Infect. 2011;17:1484–91. DOIPubMedGoogle Scholar
- Barros LB, Calil PR, Rodrigues PT, Tonini J, Fontoura PS, Sato PM, et al. Clinically silent Plasmodium vivax infections in native Amazonians of northwestern Brazil: acquired immunity or low parasite virulence? Mem Inst Oswaldo Cruz. 2022;117:
e220175 . DOIPubMedGoogle Scholar - Vafa Homann M, Emami SN, Yman V, Stenström C, Sondén K, Ramström H, et al. Detection of malaria parasites after treatment in travelers: a 12-months longitudinal study and statistical modelling analysis. EBioMedicine. 2017;25:66–72. DOIPubMedGoogle Scholar
- Fontoura PS, Macedo EG, Calil PR, Corder RM, Rodrigues PT, Tonini J, et al. Changing clinical epidemiology of Plasmodium vivax malaria as transmission decreases: Population-based prospective panel survey in the Brazilian Amazon. J Infect Dis. 2024;229:947–58. DOIPubMedGoogle Scholar
- Karunaweera ND, Ferreira MU, Hartl DL, Wirth DF. Fourteen polymorphic microsatellite DNA markers for the human malaria parasite Plasmodium vivax. Mol Ecol Notes. 2007;7:172–5. DOIGoogle Scholar
- Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology. 1999;119:113–25. DOIPubMedGoogle Scholar
- Hudson RR. Analytical results concerning linkage disequilibrium in models with genetic transformation and conjugation. J Evol Biol. 1994;7:535–48. DOIGoogle Scholar
- Francisco AP, Bugalho M, Ramirez M, Carriço JA. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinformatics. 2009;10:152. DOIPubMedGoogle Scholar
- Slater HC, Ross A, Felger I, Hofmann NE, Robinson L, Cook J, et al. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun. 2019;10:1433. DOIPubMedGoogle Scholar
- de Oliveira TC, Corder RM, Early A, Rodrigues PT, Ladeia-Andrade S, Alves JMP, et al. Population genomics reveals the expansion of highly inbred Plasmodium vivax lineages in the main malaria hotspot of Brazil. PLoS Negl Trop Dis. 2020;14:
e0008808 . DOIPubMedGoogle Scholar - Whittaker C, Slater H, Nash R, Bousema T, Drakeley C, Ghani AC, et al. Global patterns of submicroscopic Plasmodium falciparum malaria infection: insights from a systematic review and meta-analysis of population surveys. Lancet Microbe. 2021;2:e366–74. DOIPubMedGoogle Scholar
- World Health Organization. WHO Evidence Review Group on Malaria Diagnosis in Low Transmission Settings. Malaria Policy Advisory Committee Meeting Report, Session 10. 2014 [cited 2024 Jul 30]. https://www.who.int/docs/default-source/malaria/mpac-documentation/mpac-mar2014-diagnosis-low-transmission-settings-report.pdf?sfvrsn=5694f918_2.
- Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos MS, Nicolete VC, et al. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8:
e3109 . DOIPubMedGoogle Scholar - Andrade CM, Fleckenstein H, Thomson-Luque R, Doumbo S, Lima NF, Anderson C, et al. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat Med. 2020;26:1929–40. DOIPubMedGoogle Scholar
- Björkman A, Morris U. Why asymptomatic Plasmodium falciparum infections are common in low-transmission settings. Trends Parasitol. 2020;36:898–905. DOIPubMedGoogle Scholar
- Martens P, Hall L. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis. 2000;6:103–9. DOIPubMedGoogle Scholar
- Johansen IC, Rodrigues PT, Ferreira MU. Human mobility and urban malaria risk in the main transmission hotspot of Amazonian Brazil. PLoS One. 2020;15:
e0242357 . DOIPubMedGoogle Scholar - Tripura R, von Seidlein L, Sovannaroth S, Peto TJ, Callery JJ, Sokha M, et al. Antimalarial chemoprophylaxis for forest goers in southeast Asia: an open-label, individually randomised controlled trial. Lancet Infect Dis. 2023;23:81–90. DOIPubMedGoogle Scholar
- Ministry of Health of Brazil. Eliminate Malaria Brazil: national plan for the elimination of malaria [in Portuguese]. 2022 [cited 2024 Jul 30]. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/svsa/malaria/politicas-de-saude/elimina-malaria-brasil-plano-nacional-de-eliminacao-da-malaria
- Johansen IC, Ferreira MU. Unintended consequences of ‘development’ in the Amazon: commercial aquaculture and malaria in Mâncio Lima, Brazil. In: Ioris AAR, editor. Environment and development: challenges, policies and practices. London; Palgrave Macmillan: 2021. p. 387–410.
- Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar J. 2016;15:284. DOIPubMedGoogle Scholar
- Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, et al. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med. 2016;13:
e1001942 . DOIPubMedGoogle Scholar - Tadesse FG, Slater HC, Chali W, Teelen K, Lanke K, Belachew M, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. 2018;66:1883–91. DOIPubMedGoogle Scholar
- Almeida GG, Costa PAC, Araujo MDS, Gomes GR, Carvalho AF, Figueiredo MM, et al. Asymptomatic Plasmodium vivax malaria in the Brazilian Amazon: Submicroscopic parasitemic blood infects Nyssorhynchus darlingi. PLoS Negl Trop Dis. 2021;15:
e0009077 . DOIPubMedGoogle Scholar - Pan American Health Organization. Stratification of malaria based on risk of transmission and elimination of foci. Washington; The Organization: 2019 [cited 2024 Jul 30]. https://www3.paho.org/hq/index.php?option=com_docman&view=download&slug=malaria-technical-advisory-group-session-8-2019&Itemid=270&lang=en
- Fontoura PS, Finco BF, Lima NF, de Carvalho JF Jr, Vinetz JM, Castro MC, et al. Reactive case detection for Plasmodium vivax malaria elimination in rural Amazonia. PLoS Negl Trop Dis. 2016;10:
e0005221 . DOIPubMedGoogle Scholar - Fontoura PS, Silva MF, da Costa AS, Ribeiro FS, Ferreira MS, Ladeia-Andrade S, et al. Monthly biological larviciding associated with a tenfold decrease in larval density in fish farming ponds and reduced community-wide malaria incidence in northwestern Brazil. Parasit Vectors. 2021;14:445. DOIPubMedGoogle Scholar
- Holzschuh A, Lerch A, Gerlovina I, Fakih BS, Al-Mafazy AH, Reaves EJ, et al. Multiplexed ddPCR-amplicon sequencing reveals isolated Plasmodium falciparum populations amenable to local elimination in Zanzibar, Tanzania. Nat Commun. 2023;14:3699. DOIPubMedGoogle Scholar
- Schaffner SF, Badiane A, Khorgade A, Ndiop M, Gomis J, Wong W, et al. Malaria surveillance reveals parasite relatedness, signatures of selection, and correlates of transmission across Senegal. Nat Commun. 2023;14:7268. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: August 14, 2024
1Group members are listed at the end of this article.
Table of Contents – Volume 30, Number 9—September 2024
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Marcelo U. Ferreira, Institute of Biomedical Sciences, University of São Paulo, Av. Prof. Lineu Prestes 1374, 05508-900 São Paulo, Brazil
Top