Volume 30, Number 9—September 2024
Research Letter
Confirmed Case of Autochthonous Human Babesiosis, Hungary
Figure 2
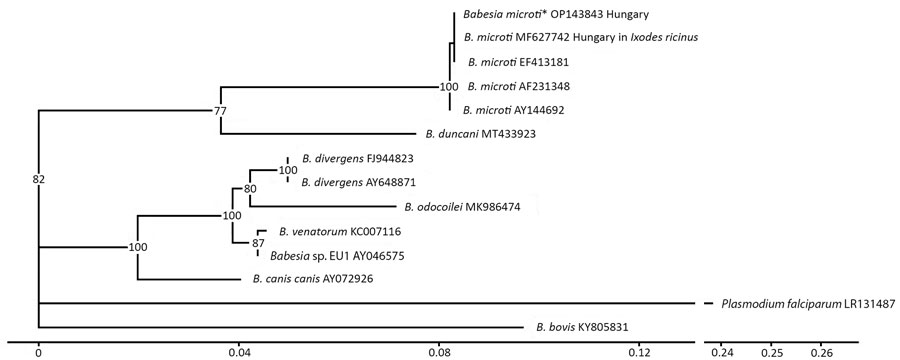
Figure 2. Phylogenetic analysis of Babesia spp. in confirmed case of autochthonous human babesiosis, Hungary. Asterisk indicates B. microti isolated from the patient in this case study. Phylogenetic tree was constructed by using Ggtree (5) according to multiple sequence alignments created by using MAFFT software (6). Best substitution model (3-parameter model, TPM2u) was selected by using functions of the phangorn version 2.11.1 R package (7) according to the Bayesian information criterion. Neighbor-joining tree was optimized by using the maximum-likelihood method. Bootstrap values were produced by 100 iterations and are indicated at branches. All data processing and plotting were performed in R version 4.4.1 (The R Project for Statistical Computing, https://www.r-project.org). GenBank accession numbers are indicated after the species name. Scale bar indicates nucleotide substitutions per site.
References
- Hildebrandt A, Zintl A, Montero E, Hunfeld KP, Gray J. Human babesiosis in Europe. Pathogens. 2021;10:1165. DOIPubMedGoogle Scholar
- Yabsley MJ, Shock BC. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int J Parasitol Parasites Wildl. 2012;2:18–31. DOIPubMedGoogle Scholar
- Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65–70.PubMedGoogle Scholar
- Moniuszko-Malinowska A, Swiecicka I, Dunaj J, Zajkowska J, Czupryna P, Zambrowski G, et al. Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect Dis (Lond). 2016;48:537–43. DOIPubMedGoogle Scholar
- Xu S, Li L, Luo X, Chen M, Tang W, Zhan L, et al. Ggtree: A serialized data object for visualization of a phylogenetic tree and annotation data. iMeta. 2022;1:
e56 . DOIPubMedGoogle Scholar - Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. DOIPubMedGoogle Scholar
- Schliep K, Potts AJ, Morrison DA, Grimm GW. Intertwining phylogenetic trees and networks. Methods Ecol Evol. 2017;8:1212–20. DOIGoogle Scholar
- Sréter T, Kálmán D, Sréterné Lancz Z, Széll Z, Egyed L. [Babesia microti and Anaplasma phagocytophilum: two emerging zoonotic pathogens in Europe and Hungary]. Orv Hetil. 2005;146:595–600.PubMedGoogle Scholar