Volume 30, Number 9—September 2024
Research Letter
Thelazia callipaeda Eyeworms in American Black Bear, Pennsylvania, USA, 2023
Cite This Article
Citation for Media
Abstract
We identified a Thelazia callipaeda eyeworm in an American black bear in Pennsylvania, USA, on the basis of its morphological features and molecular analysis. Our finding highlights emergence of a T. callipaeda worm sylvatic transmission cycle in the United States.
Thelaziosis is an emerging zoonotic disease caused by nematodes of the genus Thelazia (Spirurida, Thelazioidea). In the United States, 3 zoonotic species have been identified: Thelazia gulosa (1), T. californiensis (2), and most recently T. callipaeda (3). In Asia and Europe, T. callipaeda is considered the main agent of thelaziosis in humans, domestic animals, and wild animals (4). Over the past decade, the geographic distribution and prevalence of T. callipaeda infection has increased worldwide in scale and intensity (4). The first autochthonous case in the United States was reported in 2018 in a domestic dog (Canis lupus familiaris) from New York with a history of unilateral epiphora and blepharospasm. Since then, additional cases in domestic dogs and cats have been reported, predominately from the northeastern United States (3,5).
T. callipaeda eyeworms are found in the conjunctival sac and lacrimal duct of the definitive host. They are transmitted when a male zoophilic secretophagous Phortica variegata fly ingests first-stage larvae from the host’s lachrymal secretions. In the vector, the first-stage larvae molt to the infective third-stage larvae in the testes, migrate to the mouthparts, and are transferred to another host during subsequent feeding on lachrymal secretions (4).
The role of wildlife in the epidemiology and emergence of T. callipaeda eyeworms is not completely known. In Europe, cases of T. callipaeda eyeworm infection have been detected in a wide range of hosts, including wild carnivores, omnivores, and lagomorphs (6,7). Wild canids, particularly red foxes (Vulpes vulpes), seem to play a large role in maintaining the sylvatic cycle in thelaziosis-endemic areas of Europe (7). However, knowledge of the sylvatic transmission cycle of T. callipaeda eyeworms, along with their environmental and anthropogenic factors, remains limited. Considering the emergence of those zoonotic nematodes in non–thelaziosis-endemic areas and the need for more information about their ecology and epidemiology in the United States, we report a case of T. callipaeda eyeworm infection in an American black bear (Ursus americanus) and identify a new geographic location of transmission.
In November 2023, an adult, female American black bear was legally harvested in Coolbaugh Township, Monroe County, Pennsylvania. During processing of the bear for taxidermy preparation, multiple linear nematodes were observed behind the third eyelid. Nematodes were extracted and submitted for identification. Two additional harvested bears from Monroe and Pike Counties, Pennsylvania, were also reported to have similar ocular nematode infections, but specimens from those bears were not collected.
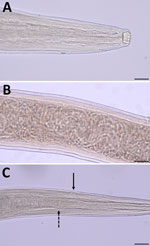
We identified 9 female and 4 male adult nematodes from the bear as T. callipaeda on the basis of morphologic and morphometric features (8). The nematodes were characterized by the presence of a cup-shaped buccal capsule and cuticular transverse striations, as well as the location of the vulvar opening anterior to the esophageal-intestinal junction on the female worms (Figure 1). Female nematodes were 1.16–1.46 cm long and 0.36–0.42 mm wide; male worms were 0.82–1.06 cm long and 0.31–0.42 mm wide. The number of transverse cuticular striations ranged from 160 to 400/mm in the cephalic, midbody, and caudal regions.
We extracted genomic DNA from a midbody fragment of a female adult worm and amplified, sequenced, and analyzed the partial cytochrome oxidase c subunit I (cox1) gene, as previously described (2). We generated a 623-bp cox1 sequence (GenBank accession no. PP739308), which showed 99%–100% maximum identity with T. callipaeda sequences available in GenBank. Phylogenetic analysis was performed by using the maximum-likelihood method and confirmed the taxonomic identification of T. callipaeda. The isolate clustered with all previous isolates from domestic animals in North America and with some isolates from Europe (Figure 2), indicating circulation of the newly introduced pathogen in wildlife habitats and transmission from domestic animals to wildlife.
The presence of adult T. callipaeda eyeworms in an American black bear suggests the establishment of a sylvatic transmission cycle in the United States and expansion of the number of definitive host species used by the zoonotic nematode. In the past decade, wild carnivores have been identified as primary definitive hosts associated with the sylvatic cycle in thelaziosis- endemic and non–thelaziosis-endemic areas of Europe and Asia (7). American black bears are the most widely distributed species of bear in North America, inhabiting diverse regions throughout Mexico, Canada, and the United States (9). Given the bears’ extensive geographic distribution and frequent and close interaction with humans and pets (10), thelaziosis in the black bear population raises concerns about the rapidly increasing incidence and geographic range of T. callipaeda eyeworms in the United States. Although further research into the extent to which black bears play a role in the maintenance of the sylvatic cycle and transmission of T. callipaeda eyeworms is needed, the presence of the zoonotic nematode in such a wide range of hosts implicates exposure and risk for transmission to threatened and endangered species and direct or indirect risk for transmission to humans and domestic animals.
Dr. Sobotyk is an assistant professor of clinical parasitology and director of the Clinical Parasitology Laboratory at the University of Pennsylvania, Philadelphia, PA. Her research focuses on zoonotic helminth infections in domestic and wild animals and improvement and development of diagnostic techniques for detecting parasitic infections of veterinary and public health relevance.
Acknowledgment
We thank the Pennsylvania Game Commission and Dillon Gruver for their continued support. We also acknowledge Shawn Lamparter’s Wildlife Design for recognition and prompting submission of the specimens.
References
- Bradbury RS, Breen KV, Bonura EM, Hoyt JW, Bishop HS. Case report: conjunctival infestation with Thelazia gulosa: a novel agent of human thelaziasis in the United States. Am J Trop Med Hyg. 2018;98:1171–4. DOIPubMedGoogle Scholar
- Sobotyk C, Foster T, Callahan RT, McLean NJ, Verocai GG. Zoonotic Thelazia californiensis in dogs from New Mexico, USA, and a review of North American cases in animals and humans. Vet Parasitol Reg Stud Reports. 2021;24:
100553 . DOIPubMedGoogle Scholar - Schwartz AB, Lejeune M, Verocai GG, Young R, Schwartz PH. Autochthonous Thelazia callipaeda infection in dog, New York, USA, 2020. Emerg Infect Dis. 2021;27:1923–6. DOIPubMedGoogle Scholar
- Otranto D, Mendoza-Roldan JA, Dantas-Torres F. Thelazia callipaeda. Trends Parasitol. 2021;37:263–4. DOIPubMedGoogle Scholar
- Manoj RRS, White H, Young R, Brown CE, Wilcox R, Otranto D, et al. Emergence of thelaziosis caused by Thelazia callipaeda in dogs and cats, United States. Emerg Infect Dis. 2024;30:591–4. DOIPubMedGoogle Scholar
- Papadopoulos E, Komnenou A, Karamanlidis AA, Bezerra-Santos MA, Otranto D. Zoonotic Thelazia callipaeda eyeworm in brown bears (Ursus arctos): A new host record in Europe. Transbound Emerg Dis. 2022;69:235–9. DOIPubMedGoogle Scholar
- Otranto D, Dantas-Torres F, Mallia E, DiGeronimo PM, Brianti E, Testini G, et al. Thelazia callipaeda (Spirurida, Thelaziidae) in wild animals: report of new host species and ecological implications. Vet Parasitol. 2009;166:262–7. DOIPubMedGoogle Scholar
- Otranto D, Lia RP, Traversa D, Giannetto S. Thelazia callipaeda (Spirurida, Thelaziidae) of carnivores and humans: morphological study by light and scanning electron microscopy. Parassitologia. 2003;45:125–33.PubMedGoogle Scholar
- Garshelis DL, Scheick BK, Doan-Crider DL, Beecham JJ, Obbard ME. The American black Bear (Ursus americanus). The IUCN Red List of Threatened Species. Washington (DC): International Union for Conservation of Nature. 2016:e.T41687A114251609.
- Di Salvo AR, Chomel BB. Zoonoses and potential zoonoses of bears. Zoonoses Public Health. 2020;67:3–13. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: August 14, 2024
Table of Contents – Volume 30, Number 9—September 2024
| EID Search Options |
|---|
|
|
|
|
|
|
![Phylogenetic relationship of Thelazia callipaeda isolate from an American black bear in Coolbaugh Township, Monroe County, Pennsylvania, USA, 2023 (GenBank accession no. PP739308), and other species of Thelazia available in GenBank (accession numbers shown). Analysis was performed by using the maximum-likelihood method (1,000 bootstrap replicates) in MEGA X version 11 (https://www.megasoftware.net). The best-fit nucleotide substitution model for the dataset was Tamura-Nei with a discrete gamma distribution, which was used to model evolutionary rate differences among sites (5 categories [+G, parameter = 0.2578]). That analysis involved 30 nucleotide sequences. There were 647 positions in the final dataset. Distances, defined as the number of nucleotide substitutions/site, were calculated by using that model. Branches corresponding to partitions reproduced in <50% of bootstrap replicates are collapsed.](/eid/images/24-0679-F2-tn.jpg)
Please use the form below to submit correspondence to the authors or contact them at the following address:
Caroline Sobotyk, University of Pennsylvania, School of Veterinary Medicine, Matthew J. Ryan Veterinary Hospital, Rm 4034, 3900 Delancey St, Philadelphia, PA 19104-6051, USA
Top