Volume 31, Number 1—January 2025
Online Report
Research and Development of Medical Countermeasures for Emerging Infectious Diseases, China, 1990–2022
Figure 2
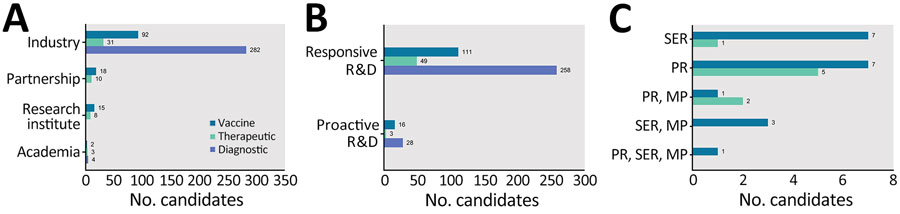
Figure 2. Pipeline of medical countermeasures for emerging infectious diseases by developer type, R&D type, and regulatory pathway, China, 1990–2022. Partnership indicates that the candidate was jointly developed by >2 developers. Responsive and proactive R&D were differentiated on the basis of the time between the occurrence of disease in China and the earliest R&D of candidate. A) Distribution of medical countermeasures by developer type and countermeasure type. B) Distribution of medical countermeasures by R&D type. C) Distribution of medical countermeasures by regulatory pathway. MP, Major Program of National Science and Technology; PR, Priority Review; R&D, research and development; SER, Special Examination Review.
Page created: November 15, 2024
Page updated: December 22, 2024
Page reviewed: December 22, 2024
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.