Volume 31, Number 1—January 2025
Research Letter
Endogenous Endophthalmitis Caused by Prototheca Microalga in Birman Cat, Spain
Cite This Article
Citation for Media
Abstract
We identified Prototheca spp. microalga in ocular samples of a cat in Spain with nontreatable endogenous endophthalmitis. Within 2 years, the eye lesions progressively worsened and neurologic signs appeared, suggesting systemic spread of the infection. On multitarget sequence analysis, the feline pathogen could not be assigned to any known Prototheca species.
Protothecosis is an uncommon disease caused by the unicellular microalga Prototheca spp., described in humans and animals and associated with systemic disease, cutaneous lesions, or both (1,2). Prototheca spp. has been identified as the cause of cutaneous lesions and in 1 case of disseminated neurologic disease in cats (2–4). Diagnosis of protothecosis can be challenging and usually is based on observation of the organism in tissues and body fluids (5). Culturing or PCR is required for a definitive diagnosis and species identification (2,4).
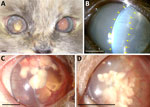
A 5-year-old female Birman cat, spayed and maintained indoors, was referred to our veterinary hospital for a 1.5-month history of uveitis in the right eye. Neuro-ophthalmic evaluation revealed that the right eye was blind and had severe signs of uveitis, whereas the left eye was unaffected. Ultrasound examination showed exudative retinal detachment in the right eye, confirming irreversible blindness. At 5.5 months, clinical signs of uveitis appeared also in the other eye (Figure, panel A). At the 6.5-month follow-up, the aqueous flare in the left eye worsened (Figure, panel B). We obtained an aqueous humor sample and a vitreous sample for cytologic examination, which revealed a mixed inflammatory process and the presence in the vitreous of structures morphologically compatible with algae of the genus Prototheca spp. (Appendix Figure 1, panel A). Antifungal therapy with itraconazole (5 mg/kg 2×/d) was initiated at 10.5 months and stopped voluntarily by the owner at 14.5 months. At the 16.5-month follow-up, the cat was completely blind, and the clinical signs had worsened with the development of corneal macro-deposits (Figure, panels C, D). At 17.5 months, we observed 2 episodes of neurologic clinical signs, including vestibular signs, ataxia, and disorientation. At 19.5 months, the owner reported that the cat had lost her hearing. At 21.5 months, the cat’s neurologic status further deteriorated, with the onset of seizures and prolonged anorexia. At this point, the owner opted for the humane euthanasia of the cat but did not give consent for a full-body necropsy.
The eyes of the cat were submitted for biopsy. The samples had been frozen before fixation and autolysis had occurred, so histopathologic investigations were challenging because of artifacts. Nevertheless, diffuse exudate was visible throughout all the ocular structures (Appendix Figure 1, panel B). We observed karyorrhectic remnants and microorganisms within the axial cornea (Appendix Figure 1, panel C). In addition, the lens capsule was ruptured, and we noted the presence of intra-lenticular microorganisms and hypermature cataract formation (Appendix Figure 1, panel D). The microorganisms exhibited a markedly periodic acid-Schiff–positive and Alcian blue–negative membrane. Results of PCR analysis for Cryptococcus spp. were negative and, on the basis of the morphology and staining characteristics, we suspected a diagnosis of Prototheca spp. infection.
The ocular samples tested positive for Prototheca spp. in PCR tests that used 3 primer sets (Appendix Table 1) and amplified a 1,800-bp sequence of the 18S rDNA, a 630-bp sequence of 28S rDNA, and a 650-bp sequence of the cytochrome B gene. We deposited the nucleotide sequences in GenBank (accession nos. PQ111814 [18S rDNA sequence], PQ122806 [28S rDNA sequence], and PQ115153 [cytochrome B gene sequence]). We conducted multitarget sequence and phylogenetic analysis by using the sequences generated in this study and cognate sequences retrieved from the National Center for Biotechnology Information database (Appendix Figure 2). The 18S rDNA, the 28S rDNA, and the cytochrome B gene sequences shared the highest nucleotide identity with Prototheca lentecrescens PK1 (GenBank accession nos. MZ198751 [86.0%], OK236514 [84.8%], and MW701399 [83.5%]) (Appendix Table 2). The feline Prototheca strain was segregated in a separate branch within the maximum-likelihood phylogenetic tree, diverging from other Prototheca species, thereby supporting the hypothesis of a distinct phylotaxonomic status for the strain SPA/2024/cat/259 (Appendix Figure 2).
Disseminated Prototheca infection already has been reported in a cat with central nervous system signs and a suspected diagnosis of multifocal lymphoma; however, in other reports, feline protothecosis has been associated with cutaneous or subcutaneous lesions (2–4). In our case, the cat had a history of chronic glucocorticoid administration for intestinal disease, which probably triggered immune suppression in the animal. In addition, the cat had received 2 fecal transplants, which might be a potential source of infection. Also, previous studies have indicated that the Birman breed is highly susceptible to certain infectious diseases, including chlamydophilosis, cryptococcosis, feline infectious peritonitis, and Tritrichomonas fetus infection (6–9). However, we could not identify the primary source of the infection because this microalga can be found in multiple environmental sources. Another limitation of our study was that we could not isolate the Prototheca strain in vitro to assess its cultural properties.
In conclusion, we describe a novel candidate Prototheca species invading the ocular tissues of a cat, a rare clinical manifestation in felids. Our findings also extend the knowledge of the genetic diversity of Prototheca spp. in animals, a piece of valuable information for pathogens with zoonotic potential.
Dr. Jimenez-Ramos is a veterinary ophthalmologist at Puchol Veterinary Hospital in Madrid, Spain. Dr. Ripolles-Garcia also is a veterinary ophthalmologist at Puchol Veterinary Hospital. Her primary research interests are retinal diseases and ocular manifestations of systemic infections.
Acknowledgments
The authors acknowledge the invaluable technical assistance provided by the staff of the Puchol Veterinary Hospital. In particular, Erica Cumpa, Patricia Leal, and Maria Peralta are to be commended for their role in coordinating the procedures and their exemplary care for the animals. Furthermore, we are grateful to Maria Soto for her invaluable assistance with sample management and study coordination.
This study was approved by the Ethics and Professional Integrity Committee of the College of Veterinary Surgeons of Madrid.
This research was funded in part by the National Laboratory for Infectious Animal Diseases, Antimicrobial Resistance, Veterinary Public Health and Food Chain Safety (grant no. RRF-2.3.1-21-2022-00001).
References
- Ahrholdt J, Murugaiyan J, Straubinger RK, Jagielski T, Roesler U. Epidemiological analysis of worldwide bovine, canine and human clinical Prototheca isolates by PCR genotyping and MALDI-TOF mass spectrometry proteomic phenotyping. Med Mycol. 2012;50:234–43. DOIPubMedGoogle Scholar
- Sykes JE. Protothecosis and chlorellosis [chapter 90]. In: Sykes JE, editor. Greene’s infectious diseases of the dog and cat, 5th edition. St. Louis: Elsevier, Inc.; 2023. p. 1126–34.
- Ely VL, Espindola JP, Barasuol BM, Sangioni LA, Pereira DB, Botton SA. Protothecosis in veterinary medicine: a minireview. Lett Appl Microbiol. 2023;76:
ovad066 . DOIPubMedGoogle Scholar - Lanave G, Pellegrini F, Palermo G, Zini E, Mercuriali E, Zagarella P, et al. Identification of Prototheca from the cerebrospinal fluid of a cat with neurological signs. Vet Sci. 2023;10:681. DOIPubMedGoogle Scholar
- Webb AA, Cullen CL. Ocular manifestations of systemic disease, part 1: the dog [chapter 37.1]. In: Gelatt KN, editor. Veterinary ophthalmology, 6th edition. Hoboken (NJ): John Wiley & Sons; 2021. p. 2329–420.
- Wills J, Howard P, Gruffydd‐Jones T, Wathes CM. Prevalence of Chlamydia psittaci in different cat populations in Britain. J Small Anim Pract. 1988;29:327–39. DOIGoogle Scholar
- Foley JE, Pedersen NC. The inheritance of susceptibility to feline infectious peritonitis in purebred catteries. Feline Pract. 1996;24:14–22.
- Golovko L, Lyons LA, Liu H, Sørensen A, Wehnert S, Pedersen NC. Genetic susceptibility to feline infectious peritonitis in Birman cats. Virus Res. 2013;175:58–63. DOIPubMedGoogle Scholar
- Kuehner KA, Marks SL, Kass PH, Sauter-Louis C, Grahn RA, Barutzki D, et al. Tritrichomonas foetus infection in purebred cats in Germany: prevalence of clinical signs and the role of co-infection with other enteroparasites. J Feline Med Surg. 2011;13:251–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: December 18, 2024
1These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 1—January 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Fernando Laguna, Department of Ophthalmology, Puchol Veterinary Hospital, 8th Sauceda St, 28050, Madrid, Spain
Top