Volume 31, Number 10—October 2025
Dispatch
Zoonotic Baylisascaris procyonis Infection in Raccoons, Mississippi, USA, 2023–2024
Cite This Article
Citation for Media
Abstract
Baylisascaris procyonis, an emerging zoonotic parasite, causes clinically significant visceral, ophthalmologic, and neurologic disease in humans. We screened raccoons (n = 46) collected from central and southern Mississippi for B. procyonis by necropsy (13.0% prevalence) and droplet digital PCR of feces (26.7% prevalence). Further surveillance to determine raccoon infection rates throughout Mississippi is indicated.
Baylisascaris procyonis roundworms are emerging zoonotic parasites that cause clinically significant visceral, ophthalmologic, and neurologic disease in humans. The 3 major categories of human Baylisascaris procyonis infection are neural larva migrans, ocular larva migrans, and visceral larva migrans (1–3). Raccoons (Procyon lotor) are the definitive host for B. procyonis roundworms (2,3). Case reports in the literature describe life-threatening neurologic disease manifestations of eosinophilic meningitis, which causes substantial illness and death (3). North American raccoons are human-habituated and thrive in urban and suburban areas, posing a serious health risk to humans (4,5).
Human B. procyonis infection occurs after ingestion of eggs from raccoon feces in contaminated soil from the environment, commonly in latrine areas in which raccoons defecate (6). Female B. procyonis roundworms produce >100,000 eggs/day/worm, and infected raccoons shed millions of eggs through fecal matter at latrine locations (1,7). Younger children and persons with developmental delays are more likely to become infected because of increased likelihood of ingesting eggs because of pica behaviors (3). In the United States, 33 cases of human B. procyonis infection have been reported (3). B. procyonis has previously been reported in states neighboring Mississippi, USA, in the southeast (5,8–10). We report the presence of B. procyonis roundworms in raccoons in Mississippi.
We collected raccoons (n = 46) during 2023–2024 from 8 different counties in central and southern Mississippi (Hancock, Harrison, Hinds, Humphreys, Lincoln, Pearl River, Yazoo, and Warren Counties). We used dog-proof traps or live traps with a scientific collection permit issued by the Mississippi Department of Wildlife Fisheries and Parks (permit no. 0207241); some collections were made from donated hunter-harvested raccoons. Raccoons were stored at −20°C until examined by necropsy. During necropsy, we examined the raccoon’s small and large intestines for the presence of B. procyonis nematodes. We collected and stored B. procyonis adult and juvenile worms in 70% ethanol before DNA analysis. At the time of necropsy, 45 of the raccoons contained fecal matter; we collected fecal samples collected from the large intestines of those animals and stored at −20°C before DNA analysis (Appendix). All animal protocols used in this study were approved by the Mississippi College Institutional Animal Care and Use Committee (protocol no. 072723) and Institutional Review Board (protocol no. 111621).
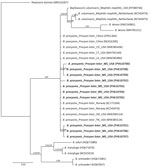
We confirmed B. procyonis presence in necropsied raccoons by morphology (Appendix Figure, panel A), Sanger sequencing and droplet digital PCR (ddPCR) analysis of fecal samples (n = 45), and sequencing of helminth DNA (n = 46) (Table; Appendix). PCR and Sanger sequencing using B. procyonis cox1, cox2, ITS1, 18s, and 28S primers revealed 100% identity to B. procyonis sequences in GenBank (accession nos. MW385486, AF179908, NC_016200, U94368, MZ092854) (11,12). We submitted DNA sequences to Genbank (accession nos. PV593814 [18s], PV594051 [28s], PV594407 [ITS1], PV600642 [cox2], and PV610700–9 [cox1]). Phylogenetic analysis of the cox1 sequences (n = 10) showed 100% identity with B. procyonis nematodes collected from raccoons in the United States, China, and Norway (Figure 1) (11,12). Necropsy of raccoons revealed B. procyonis positivity in 2/8 counties (Humphreys and Yazoo); positivity rates were >50%. The number and length of B. procyonis nematodes found in infected adult and juvenile raccoons varied (Appendix Table 1). Higher infection rates were seen in juvenile raccoons than in adult raccoons, as previously reported (1). Identification of B. procyonis eggs in fecal samples was hindered by freezing-induced morphological damage, making identification and positivity assessment difficult using traditional microscopic methods (Appendix Figure, panel B).
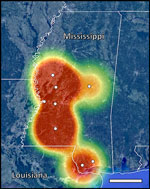
Our ddPCR analysis of fecal DNA revealed B. procyonis positivity in 6 of the 8 counties in Mississippi (Hancock, Hinds, Humphreys, Lincoln, Pearl River, and Yazoo) (Table). The ddPCR results estimated an infection rate of >50% in Hancock, Humphreys, Lincoln, and Yazoo Counties; the mean infection rate was 26.7% across all 8 counties surveyed. The mean + SE concentration of Baylisascaris sp. DNA in positive fecal samples was 2.28 + 1.84 (range 0.16–22.17) (Appendix Table 2). Heatmapping of ddPCR results estimates B. procyonis positivity in raccoons throughout large portions of central and southern Mississippi and high positivity along the Mississippi–Louisiana border (Figure 2).
We report evidence of B. procyonis roundworms in Mississippi, previously reported in neighboring states in the southeastern United States, including Louisiana, Georgia, Tennessee, and Arkansas (5,8,9,13,14). Using necropsy, we found an overall B. procyonis roundworm statewide prevalence of 13.0% across all counties tested.
Fecal analysis using ddPCR detected more B. procyonis infections than did necropsy analyses; the average statewide positivity rate was 26.7% across all counties, similar to the 31.7% fecal prevalence reported in Louisiana (5) using quantitative PCR (qPCR) techniques. ddPCR analysis indicated positive results from raccoons in Handcock, Hinds, Humphreys, Lincoln, Pearl River, and Yazoo Counties. Fecal positivity using ddPCR ranged from 17.6% to 100.0%. The limitation of convenience sampling in some counties might account for higher detection rates; only 1–3 raccoons were sampled in 4 counties. Racoon infection rates also varied by season, age, racoon population crowding, and age of animals (6,15). Overall, the infection rate estimate based on ddPCR data was higher and indicated the parasite was more widespread than was suggested by visual examination through necropsy alone. This discrepancy might be explained by the difficulty of conducting comprehensive necropsy across the entire intestine of raccoons, where some nematodes might have been overlooked; the difficulty of L3 and L4 larvae detection; or the migration of intestinal nematodes into the stomach after host death. We also found that freezing fecal samples altered B. procyonis egg morphology, making parasite eggs harder to identify using traditional microscopic methods. Overall, fecal analysis using ddPCR proved to be a simpler, more sensitive, and more comprehensive sampling strategy, supporting it as the preferred approach to estimate infection rates for this parasite of human–health concern.
The data represented in this study are limited by the small sample size from each county, and this study serves as proof of concept that this parasite is demonstrable in Mississippi racoons, indicating the need for increased provider awareness and animal surveillance because of the human pathogenic potential of this parasite. Taken together, the data estimate low prevalence across much of the surveyed range and spots of hyperendemicity. Future large-scaled surveillance efforts to more accurately estimate infection rates and fully assess the geographic range of this parasite in Mississippi are needed. Overall, our data suggest that raccoons in Mississippi harbor B. procyonis infection, representing a danger to human health.
Mr. Huerta-Beltrán is currently pursuing a master’s degree at the University of Southern Mississippi. His research primarily focuses on using molecular techniques to investigate aquatic and threatened species.
Acknowledgments
We thank the local Mississippi residents who donated raccoons to this study.
This work was supported by the Mississippi IDeA Network of Biomedical Research Excellence, funded by an Institutional Development Award from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103476. This work was also funded by Mississippi College Office of Research.
References
- Sorvillo F, Ash LR, Berlin OGW, Morse SA, Degiorgio C, Morse SA. Baylisascaris procyonis: an emerging helminthic zoonosis. Emerg Infect Dis. 2002;8:355–9. DOIGoogle Scholar
- Pai PJ, Blackburn BG, Kazacos KR, Warrier RP, Bégué RE. Full recovery from Baylisascaris procyonis eosinophilic meningitis. Emerg Infect Dis. 2007;13:928–30. DOIGoogle Scholar
- Lipton BA, Oltean HN, Capron RB, Hamlet A, Montgomery SP, Chancey RJ, et al. Baylisascaris procyonis roundworm infection in child with autism spectrum disorder, Washington, USA, 2022. Emerg Infect Dis. 2023;29:1232–5. DOIPubMedGoogle Scholar
- Gatcombe RR, Jothikumar N, Dangoudoubiyam S, Kazacos KR, Hill VR. Evaluation of a molecular beacon real-time PCR assay for detection of Baylisascaris procyonis in different soil types and water samples. Parasitol Res. 2010;106:499–504. DOIGoogle Scholar
- Straif-Bourgeois S, Cloherty E, Balsamo G, Gee L, Riegel C. Prevalence of Baylisascaris procyonis in raccoons trapped in New Orleans, Louisiana, 2014–2017. Vector Borne Zoonotic Dis. 2020;20:22–6. DOIPubMedGoogle Scholar
- Graeff-Teixeira C, Morassutti AL, Kazacos KR. Update on baylisascariasis, a highly pathogenic zoonotic infection. Vol. 29. Clin Microbiol Rev. 2016;29:375–99. DOIGoogle Scholar
- Ogdee JL, Henke SE, Wester DB, Fedynich AM. Assessing potential environmental contamination by Baylisascaris procyonis eggs from infected raccoons in southern Texas. Vector Borne Zoonotic Dis. 2017;17:185–9. DOIPubMedGoogle Scholar
- Huang MHJ, Demarais S, Brookshire WC, Strickland BK. Analysis of supplemental wildlife feeding in Mississippi and environmental gastrointestinal parasite load. Front Vet Sci. 2022;9:
995437 . DOIPubMedGoogle Scholar - Blizzard EL, Davis CD, Henke S, Long DB, Hall CA, Yabsley MJ. Distribution, prevalence, and genetic characterization of Baylisascaris procyonis in selected areas of Georgia. J Parasitol. 2010;96:1128–33. DOIPubMedGoogle Scholar
- Zhao H, Zendejas-Heredia PA, Colella V, Arguello I, Brookes K, Panicker IS, et al. Surveillance of soil-transmitted helminths and other intestinal parasites in shelter dogs, Mississippi, USA. One Health. 2024;20:
100956 . PubMedGoogle Scholar - Xie Y, Zhang Z, Niu L, Wang Q, Wang C, Lan J, et al. The mitochondrial genome of Baylisascaris procyonis. PLoS One. 2011;6:
e27066 . DOIGoogle Scholar - Nadler SA, Hudspeth DSS. Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. J Parasitol. 2000;86:380–93. DOIPubMedGoogle Scholar
- Souza MJ, Ramsay EC, Patton S, New JC. Baylisascaris procyonis in raccoons (Procyon lotor) in eastern Tennessee. J Wildl Dis. 2009;45:1231–4. DOIPubMedGoogle Scholar
- Al-Warid HS, Belsare AV, Straka K, Gompper ME. Baylisascaris procyonis roundworm infection patterns in raccoons (Procyon lotor) from Missouri and Arkansas, USA. Helminthologia. 2017;54:113–8.
- Weinstein SB. Baylisascaris procyonis demography and egg production in a California raccoon population. J Parasitol. 2016;102:622–8. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: September 25, 2025
Table of Contents – Volume 31, Number 10—October 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Scoty M. Hearst, Mississippi College, 200 S Capitol St, Clinton, MS 39056, USA
Top