Volume 31, Number 12—December 2025
Dispatch
Case of Congenital Tularemia with Neuroinvasive Disease, Utah, USA
Cite This Article
Citation for Media
Abstract
We diagnosed neuroinvasive tularemia in a neonate in Utah who had culture-negative pleocytosis in cerebrospinal fluid, rim-enhancing lesions on brain magnetic resonance imaging, and blood microbial cell-free DNA Francisella tularensis detection. Maternal history, serologic testing, and Francisella sp. identified in the fallopian tube by immunohistochemistry and 16S rRNA gene PCR strongly support congenital infection.
Tularemia, caused by Francisella tularensis, is a bacterial illness endemic to the Northern Hemisphere (1). Tularemia classically presents as ulceroglandular, glandular, oculoglandular, oropharyngeal, pneumonic, or typhoidal disease; other manifestations have been described (1–4). Neuroinvasive disease, although rare and difficult to diagnose, has also been reported (5–7).
Rarely, vertically transmitted tularemia in animals with histopathological and immunohistochemical (IHC) confirmation of F. tularensis in aborted fetuses (8,9) has been reported. One presumed case of human congenital infection has been reported. In 1947, Lide (10) reported delivery of a stillborn infant after tularemia was diagnosed in the mother. Gram-negative bacilli were observed in placental and fetal tissues without confirmatory testing (10).
We recently diagnosed congenital, neuroinvasive tularemia in a neonate after a positive blood test for microbial cell-free DNA (cfDNA) (Karius, https://kariusdx.com). IHC staining and 16S rRNA gene PCR identified F. tularensis in the mother’s fallopian tube.
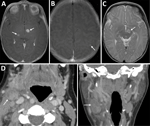
A 2-week-old infant was admitted to a hospital in Salt Lake City, Utah, USA, with lethargy, poor feeding, and pallor. The mother had ulcerative colitis in remission on infliximab therapy. One week after her infliximab dose at 34 weeks’ gestation, she experienced fever, sore throat, conjunctivitis, and cervical lymphadenopathy. After 6 days, a computed tomography scan of the neck revealed cervical lymphadenitis (Figure 1). An otolaryngologist evaluated and treated her with ceftriaxone and dexamethasone. She was evaluated by obstetrics 4 days later for decreased fetal movement. A biophysical profile score of 8 was reassuring. She underwent incision and drainage of her cervical lymph node. During the procedure, purulent fluid was encountered and sent for routine and acid-fast bacilli culture; no growth was noted at 4 days on routine culture or 42 days on acid-fast bacilli culture. She completed 10 days of amoxicillin/clavulanate.
The infant was born at 37 weeks’ gestation by cesarean section because of gestational hypertension and ongoing maternal illness. At delivery, the infant required 15 minutes of respiratory support but weaned to room air and was discharged home on day of life (DOL) 2. On DOL 16, he was seen in the emergency department for lethargy and decreased oral intake. He was tachycardic but afebrile. Complete blood count, complete metabolic panel, C-reactive protein, procalcitonin, and blood, urine, and cerebral spinal fluid (CSF) cultures were obtained; results were notable for marked peripheral leukocytosis, elevated C-reactive protein, hepatitis, and lymphocytic CSF pleocytosis (Table 1). The infant received ampicillin and ceftazidime.
Increasing lethargy and new oxygen requirement prompted transfer to the pediatric intensive care unit. At arrival, he was febrile to 39.6°C. Results of blood PCR testing for adenovirus, parvovirus B19, and cytomegalovirus were negative. A multiplex PCR panel (BioFire, https://www.biofiredx.com) detected human rhinovirus/enterovirus on a nasopharyngeal swab specimen. Results of multiplex PCR testing of the CSF (BioFire Filmarray Meningitis/Encephalitis Panel) were negative. During hospitalization, the infant developed thrombocytopenia to 115 K/μL. Bacterial blood cultures remained negative, and laboratory abnormalities improved (Table 1). He was observed for 24 hours off antibiotics, then discharged home.
He returned to the emergency department 9 days later, DOL 30. Vital signs were unremarkable, although he appeared unwell. He was transferred to our institution for further evaluation and infectious disease consultation.
Repeat lumbar puncture showed persistent lymphocytic CSF pleocytosis, and he underwent brain magnetic resonance imaging (MRI) with contrast. The MRI revealed 3 lesions, 2–7 mm in diameter, located in the left subthalamic nucleus, right thalamus, and left parietal cortex (Figure 1). Results of PCR testing of the CSF for toxoplasmosis were negative. Because of persistent illness and previously nondiagnostic evaluation, blood cfDNA testing (Karius) was performed, and results were positive for 804 molecules of microbial cfDNA/μL of F. tularensis DNA most aligned with subspecies holartica (Table 2). Treatment with intravenous (IV) ciprofloxacin and gentamicin was initiated.
The family lives on a multiple-acre property supplied by well water with nearby irrigation canals and a beaver population. Pets consisted of 1 rabbit, 2 hunting dogs, and a cat. The family had bred rabbits until ≈2 years before. The cat hunted mice and voles and was frequently in close physical contact with the mother. The cat had been “vomiting up worms” and, 2 days before the infant’s second hospitalization, was run over by a tractor and died.
On day 40 postpartum, the mother was seen by an infectious disease physician because of the infant’s tularemia diagnosis. She had continued to experience night sweats, fatigue, and anorexia, and new, diffuse arthralgias had developed, most prominently in her hands. Throat culture results were negative, but tests for F. tularensis IgG and IgM were positive (Table 2). She received 14 days of ciprofloxacin and subsequently returned to her baseline state of health.
The county health department investigated the home. The well water had increased coliform counts, but well water PCR test results for F. tularensis were negative. All other family members tested negative for F. tularensis antibodies. Their rabbit had been euthanized and was unavailable for further testing, as was the cat.
The infant received 1 week of IV gentamicin and 4 weeks of IV ciprofloxacin. Near the end of therapy, repeat microbial cfDNA testing (Karius) demonstrated a marked decline in F. tularensis microbial cfDNA levels (Table 2). Repeat brain MRI with contrast showed near complete resolution of the previous lesions with only “trace residual focus of enhancement in the left subthalamic region.” He remains well, last evaluated at 15 months of age.
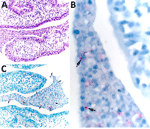
The mother underwent elective tubal ligation during her cesarean section. Extensive left-sided subacute suppurative salpingitis with serositis incidentally was noted. Once the diagnosis of congenital tularemia was suspected, remaining formalin-fixed paraffin-embedded tissue were sent to the Centers for Disease Control and Prevention, where results of an IHC assay for F. tularensis were positive for bacterial antigens with coccobacilli in the left fallopian tube, along with necroinflammatory debris (Figure 2) (11). In addition, a gram-negative bacteria 16S rRNA gene PCR performed on DNA extracts from a formalin-fixed paraffin-embedded tissue block containing tissue of the left fallopian tube was positive for Francisella spp.
We report vertical transmission of tularemia, resulting in congenital infection with neuroinvasive disease in a neonate. The findings of CSF lymphocytic pleocytosis, 3 discrete brain lesions, and positive blood cfDNA testing for F. tularensis on 2 separate samples, with resolution of brain lesions after therapy, support this diagnosis. The mother’s illness at 34 weeks’ gestation was consistent with oropharyngeal tularemia and supported by serologic testing 40 days postpartum. The detection of F. tularensis and 16S rRNA gene by PCR in the fallopian tube confirm tularemia-induced salpingitis and presumed vertical transmission.
Neuroinvasive tularemia is uncommon; lymphocytic meningitis is the most common manifestation. Rare reports exist of discrete brain lesions (5). Various antibiotic medications have been described in treatment of neuroinvasive tularemia, such as streptomycin, gentamicin, doxycycline, chloramphenicol, and ciprofloxacin, often in combination (5).
This case of vertical transmission of F. tularensis in humans, supported with microbiological confirmation by histopathological and molecular methods, is unique. Complications of tularemia in pregnancy have been reported previously (10,12–14). The source of maternal infection remains unclear. A concurrent zoonotic outbreak of tularemia among beavers occurred in neighboring counties, and the mother had multiple other potential exposures. However, we found no clear linkage (15).
This case highlights the possibility of vertical transmission of tularemia, as well as neurologic manifestations in neonates. Diagnosis can be challenging and require assistance from state health departments and specialized commercial and Centers for Disease Control and Prevention national reference laboratories.
Dr. Nelson is a pediatric infectious diseases fellow at the University of Utah. His research interests are congenital infections and clinical microbiology.
Acknowledgment
We thank Jana Ritter, Roosecelis B. Martines, and Jennifer Kasten for assisting in the pathological evaluation of the tissues and Pamela Fair and Marlene DeLeon Carnes for performing immunohistochemical and molecular assays. We also thank Sarah Park and Shivkumar Venkatasubrahmanyam for their detailed analysis of cfDNA genetic sequences.
References
- Auwaerter PG, Penn RL. Francisella tularensis (tularemia). In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 9th edition. Amsterdam: Elsevier; 2020. p. 2759–2773.
- Sharma R, Patil RD, Singh B, Chakraborty S, Chandran D, Dhama K, et al. Tularemia - a re-emerging disease with growing concern. Vet Q. 2023;43:1–16. DOIPubMedGoogle Scholar
- Seles M, Altziebler J, Gorkiewicz G, Kriegl L, Hatzl S, Ahyai S, et al. Human tularemia epididymo-orchitis caused by Francisella tularensis subspecies holartica, Austria. Emerg Infect Dis. 2023;29:2105–7. DOIPubMedGoogle Scholar
- Byington CL, Bender JM, Ampofo K, Pavia AT, Korgenski K, Daly J, et al. Tularemia with vesicular skin lesions may be mistaken for infection with herpes viruses. Clin Infect Dis. 2008;47:e4–6. DOIPubMedGoogle Scholar
- Cash-Goldwasser S, Beeson A, Marzec N, Ho DY, Hogan CA, Budvytiene I, et al. Neuroinvasive Francisella tularensis infection: report of 2 cases and review of the literature. Clin Infect Dis. 2024;78(Suppl 1):S55–63. DOIPubMedGoogle Scholar
- Blech B, Christiansen M, Asbury K, Orenstein R, Ross M, Grill M. Polyneuritis cranialis after acute tularemia infection: A case study. Muscle Nerve. 2020;61:E1–2. DOIPubMedGoogle Scholar
- Gangat N. Cerebral abscesses complicating tularemia meningitis. Scand J Infect Dis. 2007;39:258–61. DOIPubMedGoogle Scholar
- O’Toole D, Williams ES, Woods LW, Mills K, Boerger-Fields A, Montgomery DL, et al. Tularemia in range sheep: an overlooked syndrome? J Vet Diagn Invest. 2008;20:508–13. DOIPubMedGoogle Scholar
- Giannitti F, Dorsch MA, Schild CO, Caffarena RD, Sverlow K, Armién AG, et al. Pathologic and immunohistochemical evidence of possible Francisellaceae among aborted ovine fetuses, Uruguay. Emerg Infect Dis. 2023;29:141–4. DOIPubMedGoogle Scholar
- Guarner J, Zaki SR. Histopathology and immunohistochemistry in the diagnosis of bioterrorism agents. J Histochem Cytochem. 2006;54:3–11. DOIPubMedGoogle Scholar
- Fleck-Derderian S, Davis KM, Winberg J, Nelson CA, Meaney-Delman D. Systematic review of tularemia during pregnancy. Clin Infect Dis. 2024;78(Suppl 1):S47–54. DOIPubMedGoogle Scholar
- Saranovic M, Milic M, Radic I, Katanic N, Vujacic M, Gasic M, et al. Tularemia in pregnant woman, Serbia, 2018. Emerg Infect Dis. 2023;29:806–8. DOIPubMedGoogle Scholar
- Yeşilyurt M, Kiliç S, Çelebі B, Gül S. Tularemia during pregnancy: report of four cases. Scand J Infect Dis. 2013;45:324–8. DOIPubMedGoogle Scholar
- Utah Division of Wildlife Resources. DWR confirms beavers killed by disease; urges public to report any dead beavers [cited 2024 Nov 19]. https://wildlife.utah.gov/news/utah-wildlife-news/1869-dwr-confirms-beavers-killed-by-tularemia-and-urges-public-to-report-any-dead-beavers.html
Figures
Tables
Cite This ArticleOriginal Publication Date: December 12, 2025
Table of Contents – Volume 31, Number 12—December 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Brent D. Nelson, Division of Pediatric Infectious Diseases, Department of Pediatrics, University of Utah School of Medicine, 30 N Mario Capecchi Dr, Salt Lake City, UT 84112, USA
Top