Volume 31, Number 2—February 2025
Dispatch
Dengue and Other Arbovirus Infections among Schoolchildren, Haiti, 2021
Abstract
In 2021, we screened 91 children in Haiti with acute undifferentiated febrile illness for arbovirus infections. We identified a major outbreak of dengue virus type 2, with 67% of the children testing positive. Two others were positive for chikungunya East/Central/South African IIa subclade, and 2 were positive for Zika virus.
Since 2010, dengue (DENV), chikungunya (CHIKV), and Zika (ZIKV) viruses have been responsible for major outbreaks in the Caribbean region (1,2). In Haiti, reported DENV cases have tended to assume an endemic/sporadic pattern; cases surged in 2020–2022. CHIKV was responsible for epidemic disease in Haiti in 2014; ZIKV was associated with outbreaks in 2014–2016. Haiti has reported no CHIKV or ZIKV infections since 2019 (3,4). CHIKV and ZIKV are known to circulate intermittently in Brazil and other parts of South America and Central America since 2019 (3,4). Given the limitations of formal surveillance systems and arbovirus diagnostic capabilities in Haiti, we assessed the status of those viruses in Haiti to determine possible origins or evidence of persistence of viruses that might be present. We established surveillance during February–December 2021 for arboviral diseases among children attending schools operated by the Love a Child (LAC) Foundation (Fond Parisien, Haiti).
LAC schoolchildren who were brought to the LAC medical clinic for care for subjective fever without an obvious source of infection were invited to participate in the study; 91 children with febrile illness were enrolled. We collected clinical and epidemiologic data from participants and their parents and serum, nasal swab, and urine samples from case-patients, in accordance with standard protocols (5–8) (Appendix 1 Tables 1, 2). We screened serum samples by reverse transcription PCR (RT-PCR) for DENV, CHIKV, ZIKV, and Mayaro virus (MAYV) (5–8). Because COVID-19 was circulating in the community at the time of the study (9), we screened nasal swab samples from patients by RT-PCR for SARS-CoV-2. We further sequenced a random subset of 6 DENV-2–positive samples and 2 DENV-2–positive samples from patients positive for SARS-CoV-2, together with all samples positive for CHIKV, ZIKV, and SARS-CoV-2, and submitted sequences to GenBank/GISAID (Appendix 1 Table 1).
During the surveillance period, we identified a major DENV-2 outbreak (Figure 1); a total of 61 (67%) of the 91 participants had a positive serum RT-PCR result for DENV-2 virus RNA. Clinical findings were not significantly different among children who were DENV-2–positive than among those who were febrile but DENV-2–negative (Appendix 1 Table 2), underscoring the difficulties inherent in making a diagnosis of dengue fever based solely on clinical presentation. Among DENV-2–positive participants, 31% reported using mosquito nets, compared with 13% of DENV-2–negative participants (p = 0.07 by 2-tailed Fisher exact test); although not statistically significant, the trend is the opposite of what we would expect if mosquito nets were an effective tool for prevention of dengue.
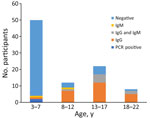
We detected CHIKV vRNA by RT-PCR in serum samples from 2 children (Appendix 1 Table 1). In contrast to the 2014 CHIKV epidemic in Haiti, infections were caused by a strain within the East/Central/South African (ECSA) IIa American subclade (7,10) (Appendix 1 Table 2). Given that we were finding active ECSA CHIKV cases by RT-PCR, we were interested in assessing the frequency with which CHIKV antibodies were present among children in our cohort. IgG seropositivity was highly associated with age (Figure 2): of the 45 children <7 years of age, 2% were seropositive for CHIKV IgG, versus 78% of children >7 years of age (p<0.001 by 2-tailed Fisher exact test). Ten children were CHIKV IgM positive but CHIKV negative on RT-PCR. Eight of those 10 children, all >7 years of age, were also IgG positive; 7/8 were positive by RT-PCR for DENV-2.
We detected ZIKV vRNA in serum samples from 2 children, 16 and 7 years of age (Appendix 1 Table 1). Both had subjective fever; the 16-year-old reported headache, arthralgias, and upper respiratory symptoms, whereas the 7-year-old noted some abdominal pain but had no other complaints. Neither had a rash. Two children (both 3 years of age) were positive for SARS-CoV-2 by nasal swab; both also were serum RT-PCR positive for DENV-2 (Appendix 1 Table 1). All RT-PCR test results for MAYV were negative.
Phylogenetic analysis revealed 2 DENV-2 clades identified in Haiti and designated as American/Asian genotype—Haiti clade 1 and 2 (Figure 3; Appendix 1; Appendix 2 Table). The DENV-2 strains from our study were clade 2, a Dominican Republic strain that initially seeded Haiti and returned in March 2020 (95% highest posterior density [HPD] June 2019–December 2020) (Figure 4). Although we could not infer directionality of spread, our analyses indicate continuous flow of DENV-2 between the 2 countries, leading to independent introductions into both.
CHIKV-positive samples from this study, together with previously reported 2016 CHIKV mosquito isolates (7), cluster with strains belonging to the ECSA IIa Brazil-Haiti subclade, isolated in Brazil in 2014 (Appendix 1 Figure 2) (2,10,11). Including the 2016 mosquito isolates, the estimated time to the most recent common ancestor was November 2015 (95% HPD October 2014–April 2016) (Appendix 1 Figure 3). Sequences of both ZIKV strains were in what was previously designated as Zika Haiti clade 1, a Haiti-Brazil lineage found in Haiti in 2014 (Appendix 1 Figure 4). Time to most recent common ancestor for the emergence of the new strains is estimated at December 2020 (95% HPD August 2018–January 2021), suggesting that ZIKV has continued to circulate in Haiti since 2014 (95% HPD October 2013–August 2014) (Appendix 1 Figure 5) (6,12).
The generalizability of our study is limited by the small sample size and the collection of all samples from a single geographic location; nonetheless, it provides a snapshot of arboviruses circulating in Haiti in 2021. We unexpectedly identified a previously unrecognized dengue type 2 outbreak among children at the LAC school, in keeping with reports from the Ministere de la Sante Publique et de la Population of a surge of DENV infections nationally (13). Work by our group and others supports the concept that DENV is endemic in Haiti. At various times, Ministere de la Sante Publique et de la Population has reported occurrence of all 4 dengue serotypes (13). Given that occurrence of severe dengue has been associated with serial infection with different DENV serotypes, one might have expected an increased likelihood of severe dengue cases in Haiti (14). However, severe dengue has been rarely reported from Haiti (13,14), and, in our experience, is uncommon among children (A. Barthelemy, unpub. data).
The sharp increase in CHIKV IgG seropositivity after age 7 we observed is consistent with infection of virtually the entire population during the initial 2014 Asian clade epidemic; children born after the 2014 epidemic appear to have had minimal exposure to the virus, as reflected in the negative IgG results. Our phylodynamic studies are consistent with introduction of the Brazil-Haiti CSA IIa CHIKV strain into Haiti around 2015, shortly after its identification in Brazil (2). What is somewhat unexpected is that this same ECSA CHIKV strain has persisted in Haiti since 2015 or earlier. The 2016 ECSA IIa strain was isolated from Aedes albopictus mosquitoes; we isolated the Asia clade from Ae. aegypti mosquitoes (7), highlighting the potential for ECSA CHIKV transmission in areas in which Ae. albopictus mosquitoes are present, but not Ae. aegypti mosquitoes. The apparently low-level persistence of ZIKV in Haiti is also of interest; the strain we initially isolated in 2014 was still present in 2021 in this study (6,12).
Warming temperatures through the Caribbean and other parts of the Americas could cause further spread of arbovirus species and increased arbovirus transmission (15); rapid spread of DENV has been documented (13). Our findings that both CHIKV and ZIKV were present but unrecognized in Haiti, coupled with limited availability of specific diagnostics, raise concern that arboviruses may spread unrecognized into other areas in the region.
Mr. Louis is an assistant researcher at the University of Florida, Emerging Pathogens Institute, where he is currently completing his PhD. His research interests focus on Vibrio cholerae, SARS-CoV-2, and arboviruses.
Acknowledgments
We thank the staff at the Love a Child Jesus Clinic for their work in providing medical care for patients included in this study.
The University of Florida institutional review board and the Comite National de Bioethique, Haiti, approved this study. We obtained signed parental informed consent and patient assent for all participants.
Studies were supported in part by National Institute of Allergy and Infectious Diseases (grant no. R21AI164007 for J.G.M. and R01AI155735 for J.G.M. and Hugh Fan, principal investigator).
References
- Pan American Health Organization. Arbovirus bulletin. Epidemiological update for dengue, chikungunya, and Zika in 2022. 2022 [cited 2024 Oct 14]. https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/annual-arbovirus-bulletin-2022.html
- de Souza WM, Ribeiro GS, de Lima STS, de Jesus R, Moreira FRR, Whittaker C, et al. Chikungunya: a decade of burden in the Americas. Lancet Reg Health Am. 2024;30:
100673 . DOIPubMedGoogle Scholar - Pan American Health Organization. PLISA Health Information Platform for the Americas—chikungunya cases. [cited 2024 Apr 17]. https://www3.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html
- Pan American Health Organization. PLISA Health Information Platform for the Americas—cases of Zika virus disease. [cited 2024 Oct 14]. https://www3.paho.org/data/index.php/en/mnu-topics/zika-weekly-en
- Ball JD, Elbadry MA, Telisma T, White SK, Chavannes S, Anilis MG, et al. Clinical and epidemiologic patterns of chikungunya virus infection and coincident arboviral disease in a school cohort in Haiti, 2014–2015. Clin Infect Dis. 2019;68:919–26. DOIPubMedGoogle Scholar
- Lednicky J, Beau De Rochars VM, El Badry M, Loeb J, Telisma T, Chavannes S, et al. Zika virus outbreak in Haiti in 2014: molecular and clinical data. PLoS Negl Trop Dis. 2016;10:
e0004687 . DOIPubMedGoogle Scholar - White SK, Mavian C, Salemi M, Morris JG Jr, Elbadry MA, Okech BA, et al. A new “American” subgroup of African-lineage Chikungunya virus detected in and isolated from mosquitoes collected in Haiti, 2016. PLoS One. 2018;13:
e0196857 . DOIPubMedGoogle Scholar - Blohm G, Elbadry MA, Mavian C, Stephenson C, Loeb J, White S, et al. Mayaro as a Caribbean traveler: Evidence for multiple introductions and transmission of the virus into Haiti. Int J Infect Dis. 2019;87:151–3. DOIPubMedGoogle Scholar
- Tagliamonte MS, Mavian C, Zainabadi K, Cash MN, Lednicky JA, Magalis BR, et al. Rapid emergence and spread of severe acute respiratory syndrome coronavirus 2 gamma (P.1) variant in Haiti. Clin Infect Dis.
- Cunha MS, Cruz NVG, Schnellrath LC, Medaglia MLG, Casotto ME, Albano RM, et al. Autochthonous transmission of East/Central/South African genotype Chikungunya virus, Brazil. Emerg Infect Dis. 2017;23:1737–9. DOIPubMedGoogle Scholar
- Giovanetti M, Vazquez C, Lima M, Castro E, Rojas A, Gomez de la Fuente A, et al. Rapid epidemic expansión of chikungunya virus East/Central/South African lineage, Paraguay. Emerg Infect Dis. 2023;29:1859–63. DOIPubMedGoogle Scholar
- Alam MM, Mavian C, Okech BA, White SK, Stephenson CJ, Elbadry MA, et al. Analysis of Zika virus sequence data associated with a school cohort in Haiti. Am J Trop Med Hyg. 2022;107:873–80. DOIPubMedGoogle Scholar
- Pan American Health Organization. PLISA Health Information Platform for the Americas—reported cases of dengue fever in the Americas [cited 2024 Apr 17]. https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-ano-en.html
- Pan American Health Organization. PLISA Health Information Platform for the Americas—dengue and severe dengue cases and deaths [cited 2024 May 10]. https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/257-dengue-casos-muertes-pais-ano-en.html
- Ryan SJ, Carlson CJ, Tesla B, Bonds MH, Ngonghala CN, Mordecai EA, et al. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob Change Biol. 2021;27:84–93. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: January 16, 2025
Table of Contents – Volume 31, Number 2—February 2025
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
J. Glenn Morris, Jr., Emerging Pathogens Institute, University of Florida, PO Box 100009, Gainesville, FL 32610-0009, USA
Top