Volume 30, Number 2—February 2024
Research
Evidence of Zika Virus Reinfection by Genome Diversity and Antibody Response Analysis, Brazil
Cite This Article
Citation for Media
Abstract
We generated 238 Zika virus (ZIKV) genomes from 135 persons in Brazil who had samples collected over 1 year to evaluate virus persistence. Phylogenetic inference clustered the genomes together with previously reported ZIKV strains from northern Brazil, showing that ZIKV has been remained relatively stable over time. Temporal phylogenetic analysis revealed limited within-host diversity among most ZIKV-persistent infected associated samples. However, we detected unusual virus temporal diversity from >5 persons, uncovering the existence of divergent genomes within the same patient. All those patients showed an increase in neutralizing antibody levels, followed by a decline at the convalescent phase of ZIKV infection. Of interest, in 3 of those patients, titers of neutralizing antibodies increased again after 6 months of ZIKV infection, concomitantly with real-time reverse transcription PCR re-positivity, supporting ZIKV reinfection events. Altogether, our findings provide evidence for the existence of ZIKV reinfection events.
Zika virus (ZIKV) is an arthropodborne virus belonging to the Flaviviridae family. Virions are enveloped for a single-stranded positive-sense RNA genome of ≈10.8-kb (1). ZIKV is transmitted through the bite of infected Aedes spp. mosquitoes, mainly A. aegypti, which are widely distributed throughout the tropical and subtropical regions of the world.
In 2015, a large ZIKV epidemic was documented in Brazil, resulting in an estimated 440,000–1.3 million cases (2). Of great concern, the epidemic was preceded by a dramatic increase in the number of congenital anomalies, including newborn microcephaly (3,4). However, since the largest outbreak in 2015, ZIKV has decreased its circulation; novel cases are only sporadically reported (5).
ZIKV infections are usually asymptomatic, although a small proportion of persons may experience mild symptoms such as fever, rash, nonpurulent conjunctivitis, muscle pain, and joint pain. During pregnancy, ZIKV infection may result in microcephaly and other congenital abnormalities in the developing fetus (3). Suspected cases are diagnosed by detection of viral RNA in blood and urine during the acute phase of the disease and in other body fluids with variable frequency and duration by reverse transcription PCR (RT-PCR) (6). As previously reported, ZIKV infection may result in a persistent viral infection, as demonstrated by the prolonged detection of viral RNA in semen; the longest detection was up to 370 days after symptom onset (7). Virus compartmentalization and persistence are common features of ZIKV infection; however, the clinical and immunological aspects of ZIKV persistence, reactivation, and reinfection are still unknown.
ZIKV phylogenetic studies have described the circulation of 2 distinct African and Asian lineages (8). The initial genetic analysis of the first ZIKV isolates from Brazil revealed the circulation of the Asian genotype during the 2015–2016 epidemic (9). Asian-derived strains that currently circulate in the Americas are now named ZIKV American strains and are well known for their capacity to infect neuronal progenitor cells, disrupting cell development, proliferation, and differentiation (10,11). Because the genomic replication of ZIKV is based on an error-prone RNA-dependent RNA polymerase (RdRp), which leads to nucleotide misincorporation during viral replication, ZIKV infection behaves as viral populations composed of genetically related sequences, similar to other RNA virus infections. As the viral replication progresses in an infected person, mutations start to accumulate, resulting in more heterogeneous viral genomic populations. Those viral population clouds are the foundation of the quasispecies theory, which posits that RNA viruses produce larger, highly variable population clouds that can evade the host immune system more efficiently (12). Furthermore, accumulating data show that viral cloud variability is able to interfere with disease progression (13,14). In this context, next-generation sequencing (NGS) provides a powerful tool to gain a deeper understanding of viral diversity by increasing the depth of sequencing coverage, defined as the number of reads for a given nucleotide). Therefore, the assessment of viral diversity is key to better understand virulence, evolution, and host-specific adaptations providing a direct translational information to mitigate effects of viral pathogens.
In this study, we deployed an NGS protocol to gain insight into the genetic diversity of ZIKV in naturally infected patients. Specifically, we used a previously established cohort study conducted in northern Brazil to assess virus diversity from patients with prolonged ZIKV infection (15–17). Since 2016, we have observed limited virus diversity and decreasing ZIKV transmission over the years, which was likely because of population immunity elicited during the first outbreak waves. We also found that virus diversity was limited in longitudinally sequenced samples from persons persistently infected with ZIKV, indicating restrained evolutionary rates and selection pressures acting on RNA arthropod-borne viruses; our results were consistent with previously published findings (18,19). However, we also detected the existence of divergent genomes within the same patient in a small number of samples analyzed; those participants responded to infection with alterations in neutralizing antibodies levels concomitantly with ZIKV redetection by real-time RT-PCR (rRT-PCR) several months after the initial virus exposure.
The study protocol and procedures have been reviewed and approved by the World Health Organization Research Ethics Review Committee (protocol ID: ERC.0002786); Brazilian National Research Ethics Commission (CAAE: 62.518.016.6.1001.0008); Institutional Ethics and Research Committee of the Evandro Chagas National Institute of Infectious Diseases, Fiocruz, Rio de Janeiro (CAAE: 62.518.016.6.2002.5262); Institutional Ethics and Research Committee of the Aggeu Magalhães Research Center, Fiocruz, Recife (CAAE: 62.518.016.6.2001.5190) and Institutional Ethics and Research Committee of the Tropical Medicine Foundation, Manaus, Amazonas (CAAE: 62.518.016.6.2003.0005).
Study Participants and Specimen Collection
Participants comprised men and women >18 years of age with a confirmed diagnosis of ZIKV infection by RT-PCR, as described previously (15,16,20). Participants were persons with symptomatic cases diagnosed at the study collaborating clinics (index case-patients) and their asymptomatic or symptomatic household and sexual contacts. After ZIKV infection confirmation performed 48 hours after study recruitment, we collected other specimens at established intervals, or visits (Table 1), and routinely tested for molecular screening using a multiplex rRT-PCR assay to detect ZIKV, dengue virus, and chikungunya virus.
NGS and Analysis
We processed all specimens with a positive ZIKV rRT-PCR result, defined as a cycle threshold (Ct) value <38, using a previously established NGS protocol (21). For this study, we processed plasma, urine, and semen samples (semen is more frequently associated with persistence). For sequencing, we first obtained a complementary DNA employing the ProtoScript II Reverse transcription kit (New England Biolabs, https://www.neb.com) and a set of random primers (random sequence [d(N)6]). We obtained whole-genome amplicons from a multiplex PCR reaction using a set of ZIKV designed primers, as described by Quick et al. (21); we purified amplicons using the Q5 High-Fidelity DNA Polymerase kit (New England Biolabs) and performed library preparation with the Nextera XT Library Prep kit (Illumina, https://www.illumina.com) using 2 ng of DNA. We sequenced the obtained libraries using the MiSeq Reagent kit version 3 (Illumina) on an Illumina MiSeq. We processed raw fastq data to generate consensus files (base calls only at regions with >5×) and to call SNVs and iSNVs (only regions with a coverage depth of >100×) using ViralFlow version 0.0.6 (22) and a reference ZIKV genome (GenBank accession no. KX197192.1).
Phylogenetic and Bayesian Analysis
The new genomic sequences reported in this study were initially submitted to a genotyping analysis using the ZIKV typing tool (http://genomedetective.com/app/typingtool/zika). We aligned genomic data generated in this study (238 genomes with coverage breadth >70 and average coverage depth of 100×) with a worldwide dataset of ZIKV genome sequences (n = 840 for all known ZIKV genotypes and n = 481 for ZIKV American strains). We aligned sequences using MAFFT (https://mafft.cbrc.jp/alignment/software) and inferred a preliminary maximum-likelihood tree using IQ-TREE version 2 (http://www.iqtree.org). Before conducting temporal analysis, we assessed our dataset for molecular clock signal in TempEst version 1.5.3 (http://tree.bio.ed.ac.uk/software/tempest) after removing any potential outliers that might violate the molecular clock assumption. To estimate a time-calibrated phylogeny, we used the Bayesian software package BEAST version 1.10.4 (https://beast.community) with the Bayesian skyline tree prior with an uncorrelated relaxed clock and the lognormal distribution. We ran analyses in duplicate in BEAST for 100 million Markov chain Monte Carlo (MCMC) steps, sampling parameters, and trees every 10,000th step. We checked convergence of MCMC chains using Tracer version 1.7.1 (https://beast.community/tracer). We summarized maximum clade credibility trees using TreeAnnotator (https://beast.community/treeannotator) after discarding 10% as burn-in. We submitted the genomes from this study to the Genome Detective for the analysis of the mutational pattern profile using the annotated genome aligner AGA (https://www.genomedetective.com/app/aga). We plotted results in R Studio version 4.2.1 (https://posit.co) using the Lollipop plot.
ZIKV Neutralization Assays
We measured ZIKV neutralizing antibody titers by a high-throughput ZIKV 50% microneutralization assay (MN50), using a wild-type live virus as described previously (22). We defined seropositivity as a titer >1:10.
Cohort Definition and Sample Assessment for NGS
During June 2017–June 2019, our study recruited a total of 255 persons with ZIKV-confirmed infection in Manaus, Brazil. Among the participants, 99% were enrolled within 1 week after the onset of illness. For this study, genomic analysis included 135 persons with confirmed ZIKV infection experiencing rash, itching, fever, and arthralgia; mean age was 38.27 (+12.97) years (Table 2). Of those 135 persons, 56 participants had >1 sample available, defined as a different specimen at the same visit (5/135) or any specimen at a different study visit (51/135). We sequenced those samples, which yielded a total of 238 ZIKV genomes with a median genome coverage breadth of 90%. Most of these genomes were obtained from plasma and urine samples; a minor proportion (n = 20) were obtained from semen specimens.
Phylogenetic Characterization
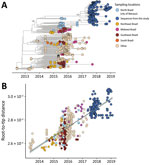
Initially, our objective was to thoroughly characterize ZIKV circulating from northern Brazil. We observed that all ZIKV strains circulating in Manaus since the beginning of the outbreak, including our newly generated genomes, grouped together in a unique clade within the ZIKV Asian lineage (Appendix 1 Figure 1). In addition, our maximum clade credibility (MCC) tree clustered our generated ZIKV genomes together with viral strains previously isolated in northern Brazil (Figure 1, panel A). From this analysis, we estimated the time of the most recent common ancestor (tMRCA) occurred in late March 2014 (95% highest posterior density range January–August 2014) (Figure 1, panel B). We also explored the collective mutational pattern found in the consensus genomes obtained in this study. Most of the mutations were observed in nonstructural protein (NS) 1 protein (5 in total) and NS5, which also has 5 mutations, although with a lower frequency than NS1 (Figure 2).
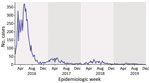
Next, we searched for the total number of ZIKV cases reported in Manaus from DATASUS (https://datasus.saude.gov.br/informacoes-de-saude-tabnet), the national health information system that compiles clinical and laboratory-confirmed cases across all the states of Brazil. Our analysis revealed that the initial occurrence of ZIKV cases in Manaus dates to 2016, a significant surge of 6,033 cases that marked the peak of the ZIKV epidemic in northern Brazil. However, after the initial surge in 2016, subsequent waves experienced a significant decrease in the overall number of reported ZIKV cases. That downward trend persisted and reached a notable low point in 2019, when only 126 cases were documented (Figure 3). We concluded that the ZIKV circulating strains in northern Brazil exhibited stability over time, undergoing minimal mutations, contributing to the decline of the epidemic.
ZIKV Within-Host Genetic Diversity
We followed our study protocol, specifically designed to investigate the persistence of ZIKV in body fluids, to assess virus diversity among persons who remained persistently infected. Although there is no consensus in the literature, we defined ZIKV persistence as any participant with ZIKV-positive rRT-PCR detection within 30 days after its initial ZIKV confirmation. By applying this criterion, we identified 10 patients who had >1 positive persistent sample from plasma, urine, or semen. Individual temporal phylogenetic analysis grouped those ZIKV-persistent genomes into 2 major clades. For 5 of the patients, all their samples grouped into a single clade in the tree (Figure 4, panel A); those clusters indicated limited viral diversity and maintenance of a single viral lineage through time in these persistently infected persons, independent of the type of specimen analyzed. Because ZIKV neutralizing antibodies (ZIKV-NAb) are highly protective and increasing titers from acute to convalescent phase are usually linked to viral clearance, we then assessed the levels of ZIKV-NAb. Our results showed that almost all the 10 persistently ZIKV-infected participants responded with higher levels (>2,000) of ZIKV-NAb by 30 days after disease onset (Figure 4, panel B), indicating a strong neutralizing antibody response at the convalescent phase. Those results eliminated the possibility of a dysregulated immune response as a cause of persistent ZIKV infections.
Our phylogenetic analysis also showed 5 participants with ZIKV genomes clustering in distinct clades or subclades in the tree (Figure 4, panel A; Figure 5; Appendix 1 Figure 2), which suggests the presence of divergent viral genomes within the same participant over time. Those participants had highly supported minor variants (approximate likelihood ratio test >70%) that were not consistently found among all samples from the same person and showed no consistent pattern of minor variant sites accumulation over time (Appendix 2). We hypothesize that the presence of such temporally divergent ZIKV genomes in the same person suggests a reinfection event by a distinct ZIKV clade. Thus, to further assess whether those participants were reinfected, we checked their rRT-PCR results. We observed that 1 participant (ID251064) had a continuous rRT-PCR–positive result up to 8 days after the initial ZIKV infection; viral RNA was not detected until study visit 8 (61–90 days after disease onset), when a ZIKV rRT-PCR result was again positive in plasma (Figure 6). Two participants (ID251069 and ID151035) tested positive for ZIKV RNA in either plasma or urine for up to 21 days after ZIKV confirmation. Those participants then remained ZIKV-negative for 10 months but returned to positivity at the last study visit, performed 311–360 days after disease onset (Figure 6). The rRT-PCR–positive samples indicating reinfection exhibited the highest degree of divergence in terms of the ZIKV genome compared with the acute phase–sequenced samples obtained from the same participants (Figure 5).
Because reports on the genomic characteristics of ZIKV isolates from 2017 onward in northern Brazil are lacking, we conducted a complementary analysis of our own dataset that revealed the presence of these exact genomes associated with reinfection within the population. Of note, we observed the presence of these same genomes in multiple samples from our cohort (Appendix 1 Figure 2), providing strong evidence that the viruses were circulating both temporally and geographically. Finally, to support the assumption of reinfection, we analyzed the levels of ZIKV-NAb at 7, 30, 180, and 360 days after disease onset, assuming that antibody titers would vary among initial infection and reinfection, mirroring rRT-PCR results. We observed that all but 1 participant (ID251069) responded with increased levels of ZIKV-NAb at the convalescent phase of the disease (30 days after symptom onset) (Figure 7). At 180 days after onset we observed a decay in ZIKV-NAb levels at an interval when the primary infection was already cleared. All 3 potentially reinfected participants (ID251069, ID151035, ID251064) responded with a second increase in the levels of ZIKV-NAb at the last interval analyzed (Figure 7), which was preceded by viral RNA redetection in plasma or urine specimens. We also discarded other arbovirus infections as an inducer of ZIKV-NAb response because our study protocol was based on a validated multiplex rRT-PCR and none of the participants tested positive for either dengue or chikungunya virus. Other well-known circulating arboviruses in northern Brazil are Oropouche virus (OROV) (23) and yellow fever virus (YFV) (24). To date, no documented reports have indicated that OROV is capable of eliciting a ZIKV-specific antibody response. Furthermore, most study participants had prior YFV vaccination, so it is unlikely that they had become infected; thus, we have effectively ruled out YFV as a potential confounding factor in relation to the antibody response associated with ZIKV reinfection. Of note, none of the 3 potential reinfection cases reported symptoms typically associated with ZIKV infection, as confirmed through a comprehensive anamnesis conducted during each study visit at our study clinic. Collectively, our data strongly support the occurrence of reinfection events in at least 3 healthy persons residing in a ZIKV-endemic area in Brazil.
Given the number of ZIKV cases registered at the peak of the 2016 epidemic in the northern and other regions of Brazil (5), added to the risk for new outbreaks, it is critical to study ZIKV evolution and its potential for adaptation to vertebrate hosts. Moreover, virus persistence may exert high evolutive pressures that contribute to virus evolution and transmission. In our study, we showed that the obtained ZIKV genomes clustered together with other ZIKV Asian strains previously isolated from northern Brazil, suggesting that this strain persisted locally through natural transmission and was kept circulating among humans until August 2019 or later. We also found that the temporal circulation of ZIKV in Manaus started a descending curve, supported by a decreasing number of cases registered after the peak of the epidemic in 2016. Thus, based on a seroprevalence study from northeastern Brazil showing that the ZIKV antibody prevalence reached a peak of 63% from 2015 to 2016 (25), in addition to other studies showing a high ( >60%) seroprevalence of ZIKV antibodies in the general population (26–28), we hypothesize that, within a single year, community immunity was enough to constrain virus circulation. In fact, our findings are consistent with a lower reproduction number (R0) since late 2016 in Salvador (25), corroborating mathematical modeling studies showing that ZIKV epidemics would be over in 3 years from its introduction in 2016 (29).
Long-term cohort studies can provide longitudinal data on individual virus diversity, virus evolution, clinical symptoms, and immunological outcomes, and so are crucial to better understanding ZIKV natural history. We found evidence of limited virus diversity over time from persistently ZIKV-infected persons, a feature that has also been observed by other independent studies (18,19). Thus, we can suggest that the evolutionary rates and selection pressures acting on ZIKV are moderate, affecting virus evolution and adaptation to local populations. In fact, similar to a previous report (30), we estimated the ZIKV whole-genome evolutionary rate at around 1.18 × 10−3 substitutions/site/year. Arboviruses primarily spread through horizontal transmission between arthropod vectors and vertebrate hosts. As a result, virus evolution is restricted by the need for optimal replication in one host, which may compromise their adaptation in the other (31), contributing to the short-term and long-term reduced number of adaptative mutations observed. In addition, various factors, including the short duration and low viremia observed in naturally infected persons (32), contribute to limiting ZIKV diversity. Consequently, our findings indicate that ZIKV displayed a relatively stable genome evolution over time and did not undergo rapid changes or diversification during the epidemic in northern Brazil.
The most notable finding of our study is the identification of reinfection events, which is highly intriguing. Given that the ZIKV epidemic in Brazil originated from a single virus strain, and combined with the observation that the virus has remained relatively stable over time, tracking reinfections becomes a challenging task. Complicating matters further, most infections are asymptomatic or cause only mild symptoms, such as fever, rash, and itching (33). As a result, persons who have been potentially reinfected may have gone unnoticed, especially considering that mild symptoms often do not prompt persons to seek medical attention. Although reinfections are extremely difficult to confirm when there are only very similar phylogenetically strains causing an outbreak, we detected divergent viruses in ZIKV-infected persons who provided longitudinal samples, which suggests a subsequent and distinct infection event.
Monitoring community virus circulation plays a crucial role in confirming infections within a population. As extensively explored for several other viruses (34), mapping diversity in a community can provide valuable information for confirming infection cases and understanding the dynamics of an outbreak. Thus, by sequencing viral genomes, it is possible to identify an specific strain or variant of the virus present in an individual or a community (35). Despite detecting the presence of these same reinfection-associated ZIKV genomes in other participants of our cohort, temporally and geographically confirming the circulation of these genomes in that population, there is a notable absence of independent studies validating the presence of these genomes at the time we detected potential reinfection cases. Most of the investigations from other groups were conducted during the early stages of the ZIKV outbreak; therefore, the literature lacks reports that describe the characteristics of the viruses circulating from late 2017 onward.
Confirmation of reinfection events based solely on molecular detection may introduce uncertainties because of the possibility of cross-contamination during sample processing. To address this concern, we conducted an assessment of ZIKV antibody response. By measuring the levels of ZIKV-NAb over time, we can add a deeper understanding of ZIKV infection dynamics, immune response effectiveness, and the potential for future reinfections (36). Here, we observed that 3 persons responded with a second increase in ZIKV-NAb levels, which was temporarily associated with rRT-PCR positivity at a late time point after the initial infection. We discarded other arbovirus infections as a cause of secondary ZIKV-NAb increase because all the participants from our cohort were tested in a multiplex rRT-PCR and none of them were positive for dengue or chikungunya virus. We also discarded YFV infection and YFV vaccination because our study participants were previously vaccinated against YFV. We also assumed that these reinfection events were very mild, mostly manifesting as an asymptomatic disease, because no symptoms were reported.
Our findings hold significant implications for public health, epidemiology, clinical practice, and diagnostics. However, the frequency of reinfections during the latest ZIKV outbreaks remains uncertain. Our study emphasizes the critical role of ongoing genomic surveillance in viral infections to enhance public health interventions. Therefore, we underscore the necessity of implementing continuous surveillance strategies, which are vital for monitoring the evolutionary changes of viruses over time and gaining a comprehensive understanding of arbovirus diversity.
Dr. Castilho has a PhD in tropical infectious diseases and works at the Tropical Medicine Foundation Doctor Heitor Vieira Dourado, Manaus, Brazil. Her research interests include arbovirus and epidemiology.
Acknowledgments
We thank the ZIKABRA Study Team: André Luiz de Abreu, Ximena Pamela Diaz Bermudez, Patrícia Brasil, Carlos Alexandre Antunes Brito, Tatiana Jorge Fernandes, Ndema Habib, Marcus Vinicius Guimarães Lacerda, Cristina Pimenta, Lydie Trautman.
Complete data set is available upon request. All the ZIKV genomes generated in this study are public available at the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home) under project no. PRJEB63302.
The research leading to these results received funding from the Wellcome Trust (grant no. 206522/Z/17/Z); UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored program executed by the World Health Organization (WHO) (WHO-SRH/HRP; grant nos. 2017/720873-0 and 2017/731359-0); Brazilian Ministry of Health (Convênio 837059/2016, Processo 25000162039201616); US National Institute of Allergy and Infectious Diseases of the National Institutes of Health: (award no. R21AI139777); and the Henry M. Jackson Foundation for the Advancement of Military Medicine (prime award no. W81XWH-18-2-0040). G.L.W. is supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico through their productivity research fellowships (no. 303902/2019-1). The funders had no role in the study design, data collection, analysis, the decision to publish, or the preparation of the manuscript.
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Author contributions: A.M.B.F., M.G., V.F., L.C.J.A., L.C.M., and C.D. performed sequencing assays and data analysis, M.C.C., C.H.A.B.-M., and A.M-N. laboratory procedures and clinical data collection; G.A.C., E.K., and N.B. performed data analysis and clinical support; R.L.B. performed microneutralization assays, K.M. performed study supervision and data analysis, G.L.W. performed phylogenetic analysis and manuscript editing, R.F.O.F. conceived and supervised the study and wrote the manuscript. All authors reviewed and approved the final version of the manuscript. The authors declare they have no conflict of interests, and the funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
References
- Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152:687–96. DOIPubMedGoogle Scholar
- Bogoch II, Brady OJ, Kraemer MUG, German M, Creatore MI, Kulkarni MA, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335–6. DOIPubMedGoogle Scholar
- Miranda-Filho DB, Martelli CM, Ximenes RA, Araújo TV, Rocha MA, Ramos RC, et al. Initial description of the presumed congenital Zika syndrome. Am J Public Health. 2016;106:598–600. DOIPubMedGoogle Scholar
- Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–8. DOIPubMedGoogle Scholar
- Yakob L. Zika virus after the public health emergency of international concern period, Brazil. Emerg Infect Dis. 2022;28:837–40. DOIPubMedGoogle Scholar
- Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, Klein L, et al. Persistence of Zika virus in body fluids—final report. N Engl J Med. 2017;379:1234–43. DOIPubMedGoogle Scholar
- Barzon L, Percivalle E, Pacenti M, Rovida F, Zavattoni M, Del Bravo P, et al. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin Infect Dis. 2018;66:1173–80. DOIPubMedGoogle Scholar
- Beaver JT, Lelutiu N, Habib R, Skountzou I. Evolution of two major Zika virus lineages: implications for pathology, immune response, and vaccine development. Front Immunol. 2018;9:1640. DOIPubMedGoogle Scholar
- Faria NR, Azevedo RDSDS, Kraemer MUG, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–9. DOIPubMedGoogle Scholar
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–90. DOIPubMedGoogle Scholar
- Nguyen HN, Qian X, Song H, Ming GL. Neural stem cells attacked by Zika virus. Cell Res. 2016;26:753–4. DOIPubMedGoogle Scholar
- Miura M, Maekawa S, Takano S, Komatsu N, Tatsumi A, Asakawa Y, et al. Deep-sequencing analysis of the association between the quasispecies nature of the hepatitis C virus core region and disease progression. J Virol. 2013;87:12541–51. DOIPubMedGoogle Scholar
- Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–8. DOIPubMedGoogle Scholar
- Calvet GA, Kara EO, Giozza SP, Bôtto-Menezes CHA, Gaillard P, de Oliveira Franca RF, et al.; ZIKABRA Study Team. Study on the persistence of Zika virus (ZIKV) in body fluids of patients with ZIKV infection in Brazil. BMC Infect Dis. 2018;18:49. DOIPubMedGoogle Scholar
- Calvet GA, Kara EO, Landoulsi S, Habib N, Bôtto-Menezes CHA, Franca RFO, et al.; ZIKABRA Study Team. Cohort profile: Study on Zika virus infection in Brazil (ZIKABRA study). PLoS One. 2021;16:
e0244981 . DOIPubMedGoogle Scholar - Calvet GA, Kara EO, Bôtto-Menezes CHA, Castilho MDC, Franca RFO, Habib N, et al. Detection and persistence of Zika virus in body fluids and associated factors: a prospective cohort study. Sci Rep. 20236;13:21557.
- Oliveira DBL, Durigon GS, Mendes EA, Ladner JT, Andreata-Santos R, Araujo DB, et al. Persistence and intra-host genetic evolution of Zika virus infection in symptomatic adults: a special view in the male reproductive system. Viruses. 2018;10:615. DOIPubMedGoogle Scholar
- Holmes EC. Patterns of intra- and interhost nonsynonymous variation reveal strong purifying selection in dengue virus. J Virol. 2003;77:11296–8. DOIPubMedGoogle Scholar
- Bôtto-Menezes CHA, Neto AM, Calvet GA, Kara EO, Lacerda MVG, Castilho MDC, et al.; ZIKABRA Study Team. Zika virus in rectal swab samples. Emerg Infect Dis. 2019;25:951–4. DOIPubMedGoogle Scholar
- Quick J, Grubaugh ND, Pullan ST, Claro IM, Smith AD, Gangavarapu K, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc. 2017;12:1261–76. DOIPubMedGoogle Scholar
- Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, De La Barrera RA, et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet. 2018;391:563–71. DOIPubMedGoogle Scholar
- Vasconcelos HB, Azevedo RS, Casseb SM, Nunes-Neto JP, Chiang JO, Cantuária PC, et al. Oropouche fever epidemic in Northern Brazil: epidemiology and molecular characterization of isolates. J Clin Virol. 2009;44:129–33. DOIPubMedGoogle Scholar
- Possas C, Lourenço-de-Oliveira R, Tauil PL, Pinheiro FP, Pissinatti A, Cunha RVD, et al. Yellow fever outbreak in Brazil: the puzzle of rapid viral spread and challenges for immunisation. Mem Inst Oswaldo Cruz. 2018;113:
e180278 . DOIPubMedGoogle Scholar - Netto EM, Moreira-Soto A, Pedroso C, Höser C, Funk S, Kucharski AJ, et al. High Zika virus seroprevalence in Salvador, northeastern Brazil limits the potential for further outbreaks. MBio. 2017;8:e01390–17. DOIPubMedGoogle Scholar
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. DOIPubMedGoogle Scholar
- Castanha PMS, Souza WV, Braga C, Araújo TVB, Ximenes RAA, Albuquerque MFPM, et al.; Microcephaly Epidemic Research Group. Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: A microcephaly case-control study in an area of high dengue endemicity in Brazil. PLoS Negl Trop Dis. 2019;13:
e0007246 . DOIPubMedGoogle Scholar - Aubry M, Teissier A, Huart M, Merceron S, Vanhomwegen J, Roche C, et al. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis. 2017;23:669–72. DOIPubMedGoogle Scholar
- Ferguson NM, Cucunubá ZM, Dorigatti I, Nedjati-Gilani GL, Donnelly CA, Basáñez MG, et al. EPIDEMIOLOGY. Countering the Zika epidemic in Latin America. Science. 2016;353:353–4. DOIPubMedGoogle Scholar
- Faria NR, Quick J, Claro IM, Thézé J, de Jesus JG, Giovanetti M, et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature. 2017;546:406–10. DOIPubMedGoogle Scholar
- Coffey LL, Vasilakis N, Brault AC, Powers AM, Tripet F, Weaver SC. Arbovirus evolution in vivo is constrained by host alternation. Proc Natl Acad Sci U S A. 2008;105:6970–5. DOIPubMedGoogle Scholar
- Fontaine A, de Laval F, Belleoud D, Briolant S, Matheus S. Duration of Zika viremia in serum. Clin Infect Dis. 2018;67:1143–4. DOIPubMedGoogle Scholar
- Roux S, Emerson JB, Eloe-Fadrosh EA, Sullivan MB. Benchmarking viromics: an in silico evaluation of metagenome-enabled estimates of viral community composition and diversity. PeerJ. 2017;5:
e3817 . DOIPubMedGoogle Scholar - Grubaugh ND, Ladner JT, Lemey P, Pybus OG, Rambaut A, Holmes EC, et al. Tracking virus outbreaks in the twenty-first century. Nat Microbiol. 2019;4:10–9. DOIPubMedGoogle Scholar
- Robbiani DF, Bozzacco L, Keeffe JR, Khouri R, Olsen PC, Gazumyan A, et al. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell. 2017;169:597–609.e11. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: January 17, 2024
1These authors contributed equally to this article.
Table of Contents – Volume 30, Number 2—February 2024
| EID Search Options |
|---|
|
|
|
|
|
|





Please use the form below to submit correspondence to the authors or contact them at the following address:
Rafael F.O. Franca, Fundacao Oswaldo Cruz Virology and Experimental Therapy, Av. Prof. Moraes Rego s/n Recife/PE 50740-465, Recife 50740465, Brazil
Top