Volume 31, Number 2—February 2025
Research Letter
Ixodes scapularis Tick Parasitizing Dog in Dawson County, Montana, USA, 2023
Cite This Article
Citation for Media
Abstract
In October 2023, a partially engorged female Ixodes tick was removed from a dog in Bozeman, Montana, USA, that had recently spent time in eastern Montana. The tick was identified as I. scapularis according to morphologic characteristics and genomic sequencing, suggesting an expanded geographic distribution requiring continued public health surveillance.
The blacklegged tick, Ixodes scapularis, is the vector of several human pathogens and the primary vector of the Lyme disease spirochete Borrelia burgdorferi in the eastern half of the continental United States (1). During the past 40 years, intensive investigations of this tick’s geographic distribution have documented its spread north and west, with new populations established in eastern North and South Dakota (2,3). Montana has remained free of I. scapularis ticks and their western counterparts, I. pacificus ticks (2). As part of a citizen science investigation during 2016–2017, a specimen of I. scapularis tick was submitted from Liberty County, Montana; however, the tick stage and host were not given, and the authors concluded that either the tick had been recently imported to the area or had been misidentified (4).
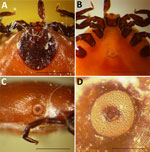
On October 12, 2023, a partially engorged female Ixodes tick was removed from the lower neck of a 7.5-year-old female French Brittany hunting dog in Bozeman, Montana, USA; the dog had recently returned with its owners from a pheasant hunting trip north of Ritchey, Dawson County, in eastern Montana. The specimen was submitted to the Schutter Diagnostic Laboratory at Montana State University in Bozeman for identification, and subsequently stored in alcohol and forwarded to the Rocky Mountain Laboratories, National Institutes of Health (Hamilton, MT, USA) for further examination. The specimen lacked the hypostome and 1 palp but, when examined microscopically, the characteristics were consistent with an I. scapularis tick (Figure 1, panels A–D). The specimen and 3 other museum specimens of unengorged female I. scapularis ticks matched I. scapularis (5,6) but not any of the 9 Ixodes spp. previously recorded in Montana (Appendix Table). The dog had not traveled outside the state before acquiring the tick, and the presumed time of attachment correlated with the trip to Dawson County (Appendix Figure 1).
Because of the condition of the specimen and potential significance of its identity, we processed the tick for genomic sequencing. We removed the tick from alcohol, froze it in liquid nitrogen, then pulverized it and extracted genomic DNA by using the MagAttract HMW DNA Kit (QIAGEN, https://www.qiagen.com) according to the manufacturer’s protocol. DNA isolated from this tick was partially degraded and produced a low yield, likely because of the long-term storage in alcohol; however, we performed whole-genome sequencing.
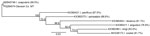
Sequencing coverage was ≈1× for the nuclear genome; the median mitochondrial genome coverage mapped to the published I. scapularis mitochondrial DNA sequence (GenBank accession no. MZ645749.1) (7). We extracted the cox1 gene consensus sequence from the data and generated a maximum-likelihood phylogeny that had 100% bootstrap support for identity between I. scapularis and this specimen. The uncorrected pairwise identity between I. scapularis and this specimen was 99.49%, whereas pairwise identity with several other Ixodes spp. established in Montana was <89% (Figure 2). We deposited the cox1 sequence into GenBank (accession no. PQ284574).
As an independent approach separate from mapping to the I. scapularis reference genome, we mapped the Illumina sequencing reads from this specimen to a database of >5 million cox1 sequences (8). The highest count (6,174,692 reads) mapped to I. scapularis, far more than the next highest count, which was I. ricinus (718,590 reads). In addition, we analyzed the microbial sequences from this tick specimen and identified 340,000 reads mapping to the I. scapularis–specific endosymbiont bacterium Rickettsia buchneri (Appendix Figure 2) (9), further supporting the identification of this tick. A relatively low number (1,509) of reads mapped to the family Borreliaceae, which includes the causative agents of Lyme disease and relapsing fever. Although this low number of reads requires independent verification, it suggests the tick specimen might have been infected with a member of the genus Borrelia.
In conclusion, the continued range expansion of I. scapularis ticks is thought to be complex and multifactorial (10). Here, we confirmed that a tick acquired in eastern Montana was I. scapularis by using 2 independent methods: morphologic characterization and genomic DNA sequence alignments. Identification is further supported by the presence of R. buchneri in the tick’s microbiome. In 2023, the Centers for Disease Control and Prevention predicted the western boundary of the I. scapularis tick range terminated in the middle of North Dakota (Appendix Figure 1). The collection of a single specimen does not signify an established population in Montana, but the public health concern regarding this tick species warrants further investigation into the potential range expansion of the I. scapularis tick. If an established I. scapularis tick population is confirmed, continued surveillance and characterization of any corresponding human pathogens will be required.
Dr. Stewart is a staff scientist at the Rocky Mountain Laboratories campus of the National Institutes of Health. His research focuses on ticks and tick-transmitted pathogens.
Acknowledgments
We thank Kimberly Ellingson Kotur for collecting and submitting the tick for identification and S.J. Tudor and Anita Mora for graphics expertise.
This work was supported, in part, by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA.
References
- Eisen RJ, Eisen L. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 2018;34:295–309. DOIPubMedGoogle Scholar
- Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol. 2016;53:349–86. DOIPubMedGoogle Scholar
- Maestas LP, Adams SL, Britten HB. First evidence of an established population of Ixodes scapularis (Acari: Ixodidae) in South Dakota. J Med Entomol. 2016;53:965–6. DOIPubMedGoogle Scholar
- Nieto NC, Porter WT, Wachara JC, Lowrey TJ, Martin L, Motyka PJ, et al. Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United States. PLoS One. 2018;13:
e0199644 . DOIPubMedGoogle Scholar - Cooley RA, Kohls GM. The genus Ixodes in North America. Natl Inst Health Bull No. 184. Washington: US Government Printing Office; 1945
- Keirans JE, Clifford CM. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. J Med Entomol Suppl. 1978;2(suppl_2):1–149. DOIPubMedGoogle Scholar
- Price DC, Brennan JR, Wagner NE, Egizi AM. Comparative hologenomics of two Ixodes scapularis tick populations in New Jersey. PeerJ. 2021;9:
e12313 . DOIPubMedGoogle Scholar - Balech B, Sandioniggi A, Marzano M, Pesole G, Santamaria M. MetaCOXI: an integrated collection of metazoan mitochondrial cytochrome oxidase subunit-I DNA sequences. Database (Oxford). 2022;2022:
baab084 . DOIPubMedGoogle Scholar - Kurtti TJ, Felsheim RF, Burkhardt NY, Oliver JD, Heu CC, Munderloh UG. Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int J Syst Evol Microbiol. 2015;65:965–70. DOIPubMedGoogle Scholar
- Telford SR III, Stewart PE, Bloom ME. Increasing risk of tick-borne disease: what should clinicians know? JAMA Intern Med. 2024;184:973–4. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: January 17, 2025
Table of Contents – Volume 31, Number 2—February 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Philip Stewart, Rocky Mountain Laboratories, NIAID, NIH, 903 South 4th St, Hamilton, MT 59840, USA
Top