Volume 31, Number 3—March 2025
Research Letter
Urban Coatis (Nasua nasua) Exposure to Alphainfluenzavirus influenzae
Cite This Article
Citation for Media
Abstract
We detected neutralizing antibodies, viral RNA, and sialic acid receptors for Alphainfluenzavirus influenzae in urban coatis (Nasua nasua) in Brazil, suggesting exposure and susceptibility. We used hemagglutination inhibition, reverse transcription quantitative PCR, and histochemistry for detection. Increased epidemiologic wildlife surveillance would improve influenza A emergency event response.
Alphainfluenzavirus influenzae, also known as as influenza A virus (IAV), continues to spread globally, causing economic loss and threatening public health (1). IAVs can infect a range of species, leading to the emergence of new subtypes with altered host tropism or virulence (2). Highly pathogenic avian influenza viruses (HPAIVs) have been detected in wild animals around the world (3). Brazil reported its first case of HPAIV in 2023, in a Thalasseus acuflavidus bird (4).
The expression of an appropriate host cell receptor that viral haemagglutinin (HA) can bind to is the key determinant of IAV ability to infect a species (5). Avian influenza viruses preferentially bind to sialic acid (SA) receptors linked to galactose by α-2,3 linkage, whereas human and classical swine influenza show preference for α-2,6 linkage. Mammal hosts that co-express both SA α-2,3 and α-2,6 receptors, primarily in the upper respiratory tract, potentially play a major role in the evolution and transmission of IAVs. Susceptibility to infection by IAVs of different origins (human, avian, or swine) can support rearrangement between IAVs and contribute to the emergence of genetically diverse viruses (6).
Coatis (Nasua nasua) are carnivores of the Procyonidae family (7). Coatis are susceptible to different virus infections, such as SARS-COV-2, and can be sentinels for animal, human, and environmental health (8). We investigated coatis IAV exposure and susceptibility from an urban park (Appendix Figure 1), which comprises an intersecting area of urban and wild environments, in Belo Horizonte, Brazil.
During 2013, 2014, 2018, 2019, and 2021, we collected samples from wild coatis. We captured coatis respecting Biosafety standards and using personal protective equipment. Ethical approvals were obtained for research development (Appendix). We placed tomahawk (Zootech, https://zootechonline.com.br/armadilhas) traps at strategic points and checked them daily. We physically examined the captured coatis and then gave each an intramuscular injection of Zoletil 100 (Virbac, https://us.virbac.com) at a dose of 7–10 mg/kg. We collected blood samples from the coatis and identified each with a subcutaneous microchip before releasing them.
We collected whole blood samples at a limit of 1% of bodyweight by jugular venipuncture from 145 coatis (Appendix Table). For 63 coatis captured in 2021, we also collected oropharyngeal swab samples and packed them in 3 mL of buffered saline solution with penicillin (200 U/mL) and streptomycin (200 μg/mL). We stored serum samples at −20°C and swabs at −80°C. We used dead coatis (n = 3) found in the park for tissue sample collection. We fixed tissues in 10% buffered formalin, embedded them in paraffin, and sectioned the tissue samples at 4 μm thickness.
We conducted hemagglutination inhibition (HI) assays to detect neutralizing antibodies to IAV (Appendix). We identified antibodies in 92.4% (n = 134) of the samples. Influenza A(H1N1)pdm09 subtype was detected in coatis’ samples from each year of the study period. H3N2 virus was detected in samples from 2018, 2019, and 2021, and seasonal human H1N1 virus was detected in 2021 (Appendix Figure 2). None of the captured coatis demonstrated any clinical manifestations of illness.
We performed RNA extraction from swabs by using QIAamp MinElute Virus Spin Kit (QIAGEN, https://www.qiagen.com), and quantitative reverse transcription PCR for universal and subtype detection of IAVs (Appendix). We detected viral RNA in 15.87% of samples from 2021 (Table). We detected subtype H3N2 genetic material from coati 347.
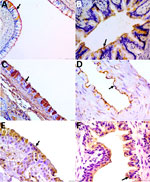
To detect α-2,6 and α-2,3 SA receptors, we selected nasal conchae, trachea, and lung tissue for lectin histochemistry technique by using Maackia amurensis and Sambucus nigra plant lectin (Appendix). We detected positive labels for those receptors in all analyzed tissues. The receptor marking was visualized as a strong brown color at the apical membrane of the nasal ephitelium and ciliated cells of the respiratory tract (nasal turbinate, trachea, bronchus and bronchiole), including globet cells, pneumocytes, and pulmonary endothelial cells (Figure). The 2 lectins labeled both receptors with diffuse distribution in the respiratory tissues.
The detection of antibodies against IAV subtypes suggests natural exposure of coatis to IAVs. We were unable to confirm the mode of IAV transmission to coatis; nevertheless, we found evidence of close contact of coatis to contaminated human waste and food, indicating the possibility of human-to-animal transmission.
The seasonal human H1N1 virus subtype, which circulated in Brazil during 2001–2003, was detected in swab samples, suggesting the possible dissemination, maintenance, and transmission capacity of coatis. Those results agree with previously published reports that detected the same viral subtype in wild carnivores during 2009–2011 (9). In 2021 and 2022, there were reports of outbreaks in Brazil triggered by the emergence of a new influenza A(H3N2) strain, named Darwin, occurring concurrently with SARS-CoV-2 as co-infection (10). The presence of α-2,6 and α-2,3 SA receptors highlight the possibility of co-infection of coatis with different viral lineages, giving the animals a potential role in IAV spillover events. Because of urban coatis’ habitats, the absence of signs of clinical illness, and the recent introduction of HPAIV into Brazil, a heightened epidemiologic wildlife surveillance strategy would improve the ability to respond to IAV emergency health events.
Dr. de Campos is a veterinarian and PhD student in animal science at Universidade Federal of Minas Gerais. Her primary research interests include virology, viral zoonoses, epidemiology, and wildlife disease.
Acknowledgments
We thank the ECOS Conservation Medicine Research Group staff for their contributions to assembling the fieldwork team and conducting laboratory analyses. We thank our colleagues and the staff at the Universidade Federal de Minas Gerais Veterinary School for their technical support, sample sharing, and statistical analysis work. We thank the Belo Horizonte Municipal Parks and Zoobotany Foundation for allowing the field work. We thank Augusto Gomes for the coatis photographs. Technical support was available from the Histopathology Laboratory of the Multidisciplinary Research Unit (MULTILAB), Department of Veterinary Clinic and Surgery, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
Financial support for this study was provided by Fundação de Amparo à Pesquisa do estado de Minas Gerais (grant no. APQ-02779-21) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Author contributions: Original draft, review and editing, data analysis: B.H.D.C., M.P.N.D.C., and R.E.; sample collection: B.H.D.C., J.S.J., M.P.N.D.C., L.R.D.A., N.S.H., and P.C.S.L.; serologic assays: B.H.D.C., G.C.F.G., E.A.C., and Z.I.P.L.; molecular testing: B.H.D.C., B.S.S., N.R.A., D.F.M.S., M.I.M.C.G., E.A.C., and Z.I.P.L.; lectin immunohistochemistry: B.H.D.C., J.S.J., M.C.L., C.I.A., and R.E.; statistical analysis: G.C.B. and C.S.F.D.O.; resources: E.A.C., Z.I.P.L., M.P.N.D.C., and R.E.; study supervision, manuscript writing, review, and editing: D.F.M.S., P.L.L.P., C.S.F.D.O., C.A.J.P., W.S.L., R.E., E.A.C., Z.I.P.L., and M.P.N.D.C. All authors have read and approved the final version of the manuscript.
References
- Jeong O-M, Kim M-C, Kim M-J, Kang H-M, Kim H-R, Kim Y-J, et al. Experimental infection of chickens, ducks and quails with the highly pathogenic H5N1 avian influenza virus. J Vet Sci. 2009;10:53–60. DOIPubMedGoogle Scholar
- Chothe SK, Bhushan G, Nissly RH, Yeh YT, Brown J, Turner G, et al. Avian and human influenza virus compatible sialic acid receptors in little brown bats. Sci Rep. 2017;7:660. DOIPubMedGoogle Scholar
- Gilbertson B, Subbarao K. Mammalian infections with highly pathogenic avian influenza viruses renew concerns of pandemic potential. J Exp Med. 2023;220:
e20230447 . DOIPubMedGoogle Scholar - Reischak D, Rivetti AV Jr, Otaka JNP, Domingues CS, Freitas TL, Cardoso FG, et al. First report and genetic characterization of the highly pathogenic avian influenza A(H5N1) virus in Cabot’s tern (Thalasseus acuflavidus), Brazil. Vet Anim Sci. 2023;22:
100319 . DOIPubMedGoogle Scholar - Lakadamyali M, Rust MJ, Zhuang X. Endocytosis of influenza viruses. Microbes Infect. 2004;6:929–36. DOIPubMedGoogle Scholar
- Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang KC. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC Vet Res. 2010;6:4. DOIPubMedGoogle Scholar
- Whiteside DP. Nutrition and behavior of coatis and raccoons. [xiii.]. Vet Clin North Am Exot Anim Pract. 2009;12:187–95, xiii. DOIPubMedGoogle Scholar
- Stoffella-Dutra AG, de Campos BH, Bastos E Silva PH, Dias KL, da Silva Domingos IJ, Hemetrio NS, et al. SARS-CoV-2 spillback to wild coatis in sylvatic-urban hotspot, Brazil. Emerg Infect Dis. 2023;29:664–7. DOIPubMedGoogle Scholar
- Bakken MA, Nashold SW, Hall JS. Serosurvey of coyotes (Canis latrans), foxes (Vulpes vulpes, Urocyon cinereoargenteus), and raccoons (Procyon lotor) for exposure to influenza A viruses in the USA. J Wildl Dis. 2020;56:953–5. DOIPubMedGoogle Scholar
- Santos CAD, Bezerra GVB, Marinho ARRAA, Sena LOC, Menezes VJ, Teixeira DCP, et al. SARS-CoV-2/influenza A (H3N2) virus coinfection: epidemiological surveillance in Northeast Brazil. Rev Soc Bras Med Trop. 2022;55:
e0132 .PubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: February 21, 2025
Table of Contents – Volume 31, Number 3—March 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Marcelo Pires Nogueira de Carvalho, Universidade Federal de Minas Gerais, Campus Pampulha da, Av. Presidente Antônio Carlos, 6627 São Luiz, Belo Horizonte, Minas Gerais 31270-901, Brazil
Top