Volume 31, Number 3—March 2025
CME ACTIVITY - Research
Efficacy and Safety of 4-Month Rifapentine-Based Tuberculosis Treatments in Persons with Diabetes
Figure 1
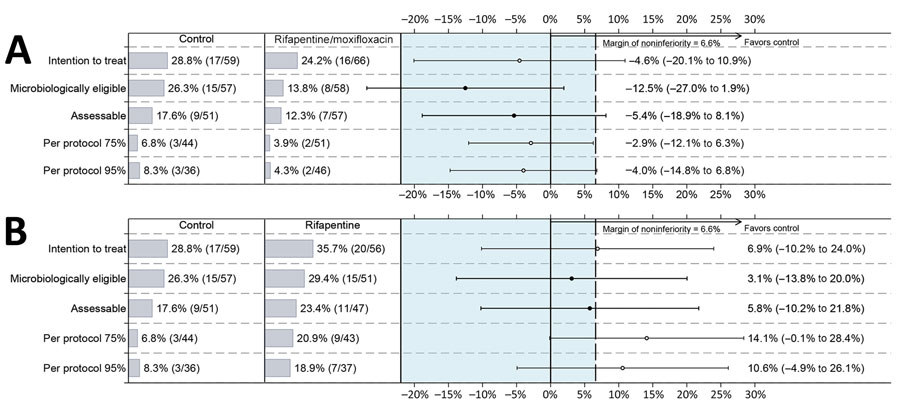
Figure 1. Unadjusted differences in unfavorable outcomes in each analysis population among participants with diabetes in a study assessing efficacy and safety of 4-month rifapentine-based tuberculosis treatments in persons with diabetes at sites in 12 countries (Brazil, Haiti, India, Kenya, Malawi, Peru, South Africa, Thailand, Uganda, United States, Vietnam, and Zimbabwe), January 2016–October 2018. Results of the efficacy results in all 5 analysis populations are shown: rifapentine/moxifloxacin regimen versus control regimen (A) and rifapentine regimen versus control regimen (B). Solid dots indicate primary results, open dots indicate secondary results, and error bars indicate 95% CIs. Dashed vertical line indicates the noninferiority margin of 6.6% for overall results in the randomized trial (18).
References
- World Health Organization. Global tuberculosis report 2023. 2023 Nov 7 [cited 2024 Aug 2]. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023
- Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. DOIPubMedGoogle Scholar
- Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff TH, et al. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis. 2007;45:428–35. DOIPubMedGoogle Scholar
- Salindri AD, Kipiani M, Kempker RR, Gandhi NR, Darchia L, Tukvadze N, et al. Diabetes reduces the rate of sputum culture conversion in patients with newly diagnosed multidrug-resistant tuberculosis. Open Forum Infect Dis. 2016;3:
ofw126 . DOIPubMedGoogle Scholar - Workneh MH, Bjune GA, Yimer SA. Diabetes mellitus is associated with increased mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients in South-Eastern Amahra Region, Ethiopia. Infect Dis Poverty. 2016;5:22. DOIPubMedGoogle Scholar
- Ma Y, Huang ML, Li T, Du J, Shu W, Xie SH, et al. Role of diabetes mellitus on treatment effects in drug-susceptible initial pulmonary tuberculosis patients in China. Biomed Environ Sci. 2017;30:671–5.PubMedGoogle Scholar
- Güler M, Unsal E, Dursun B, Aydln O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–5. DOIPubMedGoogle Scholar
- Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–46. DOIPubMedGoogle Scholar
- Jiménez-Corona ME, Cruz-Hervert LP, García-García L, Ferreyra-Reyes L, Delgado-Sánchez G, Bobadilla-Del-Valle M, et al. Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax. 2013;68:214–20. DOIPubMedGoogle Scholar
- Restrepo BI, Schlesinger LS. Impact of diabetes on the natural history of tuberculosis. Diabetes Res Clin Pract. 2014;106:191–9. DOIPubMedGoogle Scholar
- Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171–85. DOIPubMedGoogle Scholar
- Martinez N, Smulan LJ, Jameson ML, Smith CM, Cavallo K, Bellerose M, et al. Glycerol contributes to tuberculosis susceptibility in male mice with type 2 diabetes. Nat Commun. 2023;14:5840. DOIPubMedGoogle Scholar
- Alfarisi O, Mave V, Gaikwad S, Sahasrabudhe T, Ramachandran G, Kumar H, et al. Effect of diabetes mellitus on the pharmacokinetics and pharmacodynamics of tuberculosis treatment. Antimicrob Agents Chemother. 2018;62:e01383–18. DOIPubMedGoogle Scholar
- Chiang CY, Bai KJ, Lin HH, Chien ST, Lee JJ, Enarson DA, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. 2015;10:
e0121698 . DOIPubMedGoogle Scholar - Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, et al.; AIDS Clinical Trials Group; Tuberculosis Trials Consortium. Tuberculosis Trials Consortium. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med. 2021;384:1705–18. DOIPubMedGoogle Scholar
- Carr W, Kurbatova E, Starks A, Goswami N, Allen L, Winston C. Interim guidance: 4-month rifapentine-moxifloxacin regimen for the treatment of drug-susceptible pulmonary tuberculosis—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:285–9. DOIPubMedGoogle Scholar
- World Health Organization. Treatment of drug-susceptible tuberculosis: rapid communication. 2021 Jun 14 [cited 2024 Aug 2]. https://www.who.int/publications/i/item/9789240028678
- Lagerlund O, Strese S, Fladvad M, Lindquist M. WHODrug: a global, validated and updated dictionary for medicinal information. Ther Innov Regul Sci. 2020;54:1116–22. DOIPubMedGoogle Scholar
- National Clinical Trials Network. Common Terminology Criteria for Adverse Events (CTCAE). 2021 Apr 19 [cited 2024 Aug 2]. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- US Food and Drug Administration. Population pharmacokinetics: guidance for industry. 2022 Feb 3 [cited 2024 Aug 2]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics
- Magee MJ, Salindri AD, Kyaw NTT, Auld SC, Haw JS, Umpierrez GE. Stress hyperglycemia in patients with tuberculosis disease: epidemiology and clinical implications. Curr Diab Rep. 2018;18:71. DOIPubMedGoogle Scholar
- International Diabetes Federation. International Diabetes Federation diabetes atlas. 10th edition. 2021 [cited 2024 Aug 2]. https://diabetesatlas.org/atlas/tenth-edition
1Members of group listed at the end of this article.
2Members of group listed at the end of this article.