Volume 31, Number 4—April 2025
Research Letter
Yellow Fever Virus in Mosquitoes from Rainforest Bordering Manaus, Brazil, 2022
Abstract
We detected yellow fever virus in Haemagogus mosquitoes collected in 2022 in an Amazon rainforest bordering Manaus, Brazil. The viral genome sequence occupied a basal position within the South American I genotype 1E lineage. Our findings reinforce the Amazon Basin as a source for yellow fever virus re-emergence.
In Brazil, yellow fever virus (YFV) is transmitted in a sylvatic cycle between neotropical monkeys and canopy-dwelling Haemagogus and Sabethes spp. mosquitoes, occasionally spilling over into humans by way of bridge vectors (i.e., mosquitoes that bite both hosts) (1). Mandatory yellow fever (YF) vaccination combined with mosquito control initiatives have effectively eradicated the urban YF cycle; the last reported urban outbreak occurred in 1942 (2). Endemic to the Amazon Basin, the YFV sylvatic cycle remains the main reservoir for YFV spillover in Brazil and was the source of the 2016–2021 YF epidemic, which expanded far east and south of the basin (2,3). Because sylvatic cycles are largely impervious to human intervention and eradication (2), surveillance is crucial in identifying areas at risk for virus spillover.
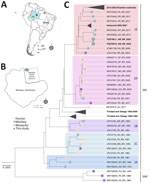
We conducted an entomological survey from May 2021 through June 2022 at the Adolpho Ducke Forest Reserve, 100 square kilometers of primary rainforest bordering Manaus in Amazonas state, Brazil (Figure, panels A, B; Appendix Figure). We sampled mosquitoes at ground level and on 5-meter platforms with hand nets and aspirators as part of ongoing studies investigating mosquito communities at forest edges (4). We morphologically identified a subsample of 687 female Haemagogus mosquitoes as Haemagogus janthinomys (81%), Hg. leucocelaenus (12%), Hg. baresi (6%), or Haemagogus spp. (1%). We screened mosquitoes in pools of ≤10 specimens, grouped by species, site, height, and collection date (n = 378 pools; mean 1.496 [SD 1.162] specimens/pool; ) (Appendix).
We macerated samples, extracted RNA, and performed YFV-specific quantitative reverse transcription PCR (5). Two pools of single Hg. janthinomys mosquitoes collected at a treefall gap and 1 Haemagogus sp. mosquito collected in undisturbed forest tested positive for YFV (Figure, panel B). We morphologically identified the unclassified mosquito as Hg. baresi, but DNA barcoding showed its COI (cytochrome c oxidase subunit 1) sequence (GenBank accession no. PQ247126) to be 99.99% identical to a Hg. janthinomys reference sequence (accession no. NC_028025.1) as well as to the 2 morphologically identified Hg. janthinomys samples (accession nos. PQ247125 and PQ247127). Lacking a published COI barcode for Hg. baresi to contextualize this finding, we referred to this mosquito specimen as Hg. sp.
All positive samples were from those collected at ground level, 500 meters interior to the forest edge, and at the end of the rainy season, in May and June 2022. We used a maximum-likelihood method to estimate infection rates of 4.37 (95% CI 1.088–11.28) per 1,000 Haemagogus mosquitoes (Appendix).
We performed Illumina next-generation sequencing (https://www.illumina.com) and obtained 1 complete and 1 near-complete genome from RNA extracted from the 2 Hg. janthinomys samples and a partial NS1 fragment (485 bp) from the Hg. sp. sample. We conducted genotyping using a yellow fever typing tool, which placed the sequences in the South American I (SAI) genotype. Phylogenetic analysis of the genomes sequenced placed this sequence in a basal position within the 1E lineage, closely related to sequences from the midwest region of Brazil, although not clustering in clades associated with recent YF outbreaks (2016–2022) (Figure, panel C; Appendix). We isolated YFV from this sample in C6/36 cells, which exhibited cytopathic effects (cell lysis) 6 days postinfection, confirming virus viability (Appendix).
Our study data confirm circulation of YFV near Manaus in forest-dwelling Hg. janthinomys mosquitoes, a sylvatic vector implicated in the most recent YF outbreaks in Brazil (6). Haemagogus mosquitoes feed primarily on monkeys but will also feed on humans (6), particularly at forest edges (4). Detection of YFV in rainforests bordering rural and periurban areas is concerning from a public health standpoint because of the comingling of humans, wildlife, and periurban and forest mosquitoes in such settings, creating the potential for arbovirus spillover (1). The risk of YF reurbanization remains a paramount concern given the widespread infestation of Ae. aegypti mosquitoes throughout South America (2). To date, high YFV vaccination coverage in Amazonas state (7) has averted outbreaks in the region, but vaccine uptake is declining (8). The YFV genomes we sequenced did not cluster with sequences from outbreaks in Brazil occurring in 2016–2021 and did not have the signature 9 amino acid motif associated with the latest outbreak (9).
Our findings emphasize the critical role of the Amazon Basin in maintaining and disseminating YFV strains to other regions of Brazil (10) and to neighboring countries. We sequenced the complete YFV genome from 1 Hg. janthinomys sample, a considerable achievement given the scarcity of genome data from the north and midwest regions of Brazil. Whole-genome sequences are crucial to understanding YFV migration dynamics in these regions (10). Unfortunately, we could not isolate and sequence the whole genome from the remaining 2 Haemagogus samples because of low viral loads, indicated by high quantitative reverse transcription PCR cycle threshold values (34.0 and 36.0). Our findings demonstrate that vector surveillance provides an early warning for arbovirus circulation, identifies high-risk areas for pathogen spillover, and guides public health efforts (vector control and vaccination) to mitigate future outbreaks.
Ms. Bernardi is a biomedical and a Master student of São José do Rio Preto School of Medicine, São José do Rio Preto, Brazil. Her research interests involve virus surveillance in mosquitoes captured in the Brazilian Amazon. Ms. Sacchetto is a virologist funded by The Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics. Her research interests involve emerging virus genomic surveillance and characterization.
Acknowledgment
The sequences obtained in this study are available in GenBank (accession nos. PQ247125–7 [COI] and PQ276810–2 [YFV]).
References
- Hanley KA, Monath TP, Weaver SC, Rossi SL, Richman RL, Vasilakis N. Fever versus fever: the role of host and vector susceptibility and interspecific competition in shaping the current and future distributions of the sylvatic cycles of dengue virus and yellow fever virus. Infect Genet Evol. 2013;19:292–311. DOIPubMedGoogle Scholar
- Sacchetto L, Drumond BP, Han BA, Nogueira ML, Vasilakis N. Re-emergence of yellow fever in the neotropics - quo vadis? Emerg Top Life Sci. 2020;4:399–410.PubMedGoogle Scholar
- Rezende IM, Sacchetto L, Munhoz de Mello É, Alves PA, Iani FCM, Adelino TER, et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl Trop Dis. 2018;12:
e0006538 . DOIPubMedGoogle Scholar - Hendy A, Fé NF, Pedrosa I, Girão A, Figueira Dos Santos TN, Mendonça CR, et al. Forest edge landscape context affects mosquito community composition and risk of pathogen emergence. iScience. 2024;28:
111576 . DOIPubMedGoogle Scholar - Domingo C, Patel P, Yillah J, Weidmann M, Méndez JA, Nakouné ER, et al. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J Clin Microbiol. 2012;50:4054–60. DOIPubMedGoogle Scholar
- Abreu FVS, Ribeiro IP, Ferreira-de-Brito A, Santos AACD, Miranda RM, Bonelly IS, et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016-2018. Emerg Microbes Infect. 2019;8:218–31. DOIPubMedGoogle Scholar
- Shearer FM, Moyes CL, Pigott DM, Brady OJ, Marinho F, Deshpande A, et al. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect Dis. 2017;17:1209–17. DOIPubMedGoogle Scholar
- The Municipal Health Department (Semsa Manaus). Prefeitura alerta para baixa cobertura da vacinação contra febre amarela em Manaus [cited 2023 May 9]. https://www.manaus.am.gov.br/semsa/prefeitura-alerta-para-baixa-cobertura-da-vacinacao-contra-febre-amarela-em-manaus
- Delatorre E, de Abreu FVS, Ribeiro IP, Gómez MM, Dos Santos AAC, Ferreira-de-Brito A, et al. Distinct YFV lineages co-circulated in the central-western and southeastern Brazilian regions from 2015 to 2018. Front Microbiol. 2019;10:1079. DOIPubMedGoogle Scholar
- Giovanetti M, Pinotti F, Zanluca C, Fonseca V, Nakase T, Koishi AC, et al. Genomic epidemiology unveils the dynamics and spatial corridor behind the Yellow Fever virus outbreak in Southern Brazil. Sci Adv. 2023;9:
eadg9204 . DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: March 17, 2025
1These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 4—April 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Maurício L. Nogueira, Faculdade de Medicina de São José do Rio Preto, Av Brigadeiro Faria Lima 5416, 1509-000, Vila São Pedro, São José do Rio Preto, São Paulo, Brazil
Top