Volume 31, Number 4—April 2025
Dispatch
Alpha-Gal Syndrome after Ixodes scapularis Tick Bite and Statewide Surveillance, Maine, USA, 2014–2023
Cite This Article
Citation for Media
Abstract
In the United States, alpha-gal syndrome (AGS) is primarily associated with lone star tick (Amblyomma americanum) bites. We describe AGS onset after an Ixodes scapularis tick bite and present AGS surveillance in Maine, 2014–2023. US health and public health professionals should be aware of AGS outside the established lone star tick range.
Alpha-gal syndrome (AGS) is an IgE-mediated hypersensitivity to the disaccharide galactose-α-1,3-galactose (α-gal), found in tissues of nearly all mammals. Spatial distribution of AGS case-patients in the United States closely follows the geographic range of established lone star tick (Amblyomma americanum) populations. However, some case-patients were identified outside that range (1,2). Other global tick species cause AGS in humans; other human-biting ticks in the United States may play a role in α-gal sensitization. The α-gal molecule is found in saliva or salivary glands of Haemaphysalis longicornis and Ixodes scapularis ticks (3,4).
To examine the possible role of other tick species causing AGS in the United States, we investigated a patient with confirmed AGS in Maine, USA, showing symptoms 9 days after a blacklegged tick (I. scapularis) bite. The Maine Center for Disease Control and Prevention (Maine CDC) collected surveillance data for 57 confirmed or suspect cases of AGS, including this case.
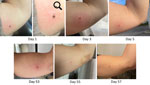
On May 4, 2022, a 45-year-old woman discovered an attached tick medial to her left bicep after returning from a wooded path in York County, Maine (Figure 1, panel A). She appropriately removed the tick and identified it as an adult female I. scapularis, which entomologists at the Centers for Disease Control and Prevention (US CDC) confirmed through morphologic and molecular identification (5). By May 7, 2022, the bite area had become inflamed and pruritic and had an enlarged, erythematous circumference.
The patient was otherwise healthy and had no allergies and no known recent exposure to endoparasites or ectoparasites (e.g., mites) except the reported tick; she was treated 9 years earlier for presumed ascariasis (records not available) attributed to organic farm work in Maine. At the time of the tick bite, she worked indoors and participated in regular outdoor recreation in Maine. She traveled to suburban Fort Lauderdale, Florida, USA, 112 days before the tick bite and reported no known tick bites while traveling. She had not previously found an attached tick in >10 years.
The patient’s first gastrointestinal (GI) symptoms occurred May 13, 2022, nine days after the tick bite and 2.5 hours after a meal of roasted rabbit and 1 alcoholic beverage. Symptoms, including delayed-onset abdominal pain and malaise lasting ≈3 hours, continued to occur over the next 2 weeks after meals containing red meat (Table 1). All meals were shared with others who did not experience symptoms, and only mammalian meat products were associated with symptoms. A severe episode of diarrhea and vomiting hours after beef consumption prompted the patient to visit a healthcare provider (HCP) 20 days after the tick bite.
The HCP ordered a complete blood count, comprehensive metabolic panel, lipase, amylase, and Helicobacter pylori breath test on May 24, 2022, to investigate the patient’s abdominal pain. All results were unremarkable. An ultrasound for gallstones was ordered but not completed because results of serum α-gal–specific IgE testing showed levels greater than the upper limit of detection at >100 kU/L (Eurofins Viracor, https://www.eurofins-viracor.com) (Figure 2). The HCP advised avoiding beef, pork, and lamb. The patient continued to consume and tolerate small amounts of dairy, including cheese and half and half. Ice cream and milkshakes caused delayed mild GI distress and nausea. Symptoms resolved on an avoidance diet.
The patient’s symptoms fit the GI phenotype of AGS (6). She did not experience hives, angioedema, respiratory difficulty, hypotension, or anaphylaxis. Meal cofactors, such as alcohol and exercise, might have accentuated symptoms (7).
The patient found 2 ticks attached in the 1–2 months after initial tick attachment: nymphal I. scapularis (US CDC morphologic and molecular confirmation) and Dermacentor variabilis (patient identification, specimen not available). The nymphal I. scapularis bite site became mildly pruritic, but the D. variabilis bite site did not. The original tick bite site remained pruritic for 2 months.
Consumption of bacon at 3 months and steak at 7 months after first symptoms led to delayed heartburn sensations. Ten months after first symptoms, the patient tolerated a steak dinner and roast beef sandwich and resumed eating red meat. α-gal–specific IgE at 13 months (June 13, 2023) measured 16 kU/L and at 25 months measured 4.58 kU/L.
Although AGS is not a reportable condition in Maine, Maine CDC increased surveillance efforts in 2023 to elucidate epidemiologic trends in locally reported cases (Table 2). Maine CDC received positive α-gal–specific IgE laboratory reports spanning November 2014–October 2023 for 57 Maine residents (average age 57 years; range 7–81 years), including 35 (61%) men and 22 (39%) women; 29 (51%) were 45–64 years of age, and 20 (36%) were >65 years of age. Of the 57 case-patients, 55 had race and ethnicity data available and reported White race and non-Hispanic ethnicity. Case-patients resided in 12 counties; 74% lived in coastal counties (Table 2).
Maine CDC confirmed AGS in 23 of 57 case-patients according to the 2022 Council of State and Territorial Epidemiologists case definition by interviewing HCPs or patients and reviewing medical charts. The other 34 case-patients were categorized as suspected cases. Those cases were not confirmed because of insufficient information in medical charts and absence of patient interviews. Insufficient information took 1 of 2 forms: not enough information to confirm allergy (11/57 case-patients) or evidence of red meat allergy noted but without delayed timing of symptoms specified (23/57 case-patients). For the 23 case-patients without delayed timing of symptoms specified, chart review revealed clinical evidence of allergy to meat or α-gal–containing products, including 7 case-patients with clinician-diagnosed AGS.
Interviews were conducted with 12 of 23 confirmed case-patients and travel histories collected from 10. Regarding symptom onset date, 3 of 10 reported and 7 of 10 denied out-of-state travel in the prior 6 months, but 4 of 7 who denied proximate travel reported out-of-state travel or residence in prior years. Of 12 case-patients interviewed, 11 reported >1 recent tick bites. Interviewed case-patients self-reported exposures to A. americanum, I. scapularis, and other ticks. Chart review and HCP interviews did not capture travel or tick exposures for the 11 confirmed case-patients not interviewed.
We report a confirmed case of AGS in a Maine resident with symptom onset 9 days after a confirmed bite from an I. scapularis tick. Although lone star ticks have been sporadically found in Maine since the 1990s, established populations have not been identified (8). Lone star ticks comprised 0.5% of specimens submitted to the University of Maine Cooperative Extension Tick Laboratory in 2022 (9). Of 95 lone star tick submissions since 2019, at least 20% were reportedly acquired in Maine (10). The patient described here was bitten early in tick season, and the bite produced a pronounced cutaneous reaction often described in AGS, suggesting the I. scapularis tick bite on May 4, 2022, was associated with AGS onset (7,11,12).
A prior study found that patients with AGS report greater numbers and frequency of tick bites than do negative controls (13). The patient might have experienced other lifetime tick bites, some unrecognized, that contributed to AGS development. Prior α-gal sensitization from other lifetime tick bites or prior Ascaris roundworm exposure (associated with α-gal sensitization globally) might also explain the rapid 9-day symptom development (14,15). Nine days is within a range observed in coauthor experiences diagnosing and managing patients with AGS (S.P. Commins, unpub. data).
In conclusion, we confirmed AGS in 23 Maine residents identified through retrospective surveillance. Further exploration is necessary regarding the role of I. scapularis ticks in AGS and factors driving AGS onset in patients residing outside the established lone star tick range. Our findings highlight the need for HCPs and public health professionals in such regions to be aware of AGS.
Dr. Saunders is an infectious diseases clinical fellow at the University of North Carolina at Chapel Hill. Her current research includes the epidemiology of alpha-gal syndrome.
Acknowledgments
We thank the patient for her generosity in supporting the publication of this case report, and we thank Paul Mead and Alison Hinckley for their review.
This project was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and Centers for Disease Control and Prevention (E.F.S.).
S.P.C. is on the speaker’s bureau of Genentech, received a grant from Revivicor, and receives royalties from UptoDate. All other authors have no conflicts of interest to report.
References
- Platts-Mills TAE, Commins SP, Biedermann T, van Hage M, Levin M, Beck LA, et al. On the cause and consequences of IgE to galactose-α-1,3-galactose: A report from the National Institute of Allergy and Infectious Diseases Workshop on Understanding IgE-Mediated Mammalian Meat Allergy. J Allergy Clin Immunol. 2020;145:1061–71. DOIPubMedGoogle Scholar
- Thompson JM, Carpenter A, Kersh GJ, Wachs T, Commins SP, Salzer JS. Geographic distribution of suspected alpha-gal syndrome cases—United States, January 2017–December 2022. MMWR Morb Mortal Wkly Rep. 2023;72:815–20. DOIPubMedGoogle Scholar
- Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, et al. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol. 2019;10:1056. DOIPubMedGoogle Scholar
- Chinuki Y, Ishiwata K, Yamaji K, Takahashi H, Morita E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy. 2016;71:421–5. DOIPubMedGoogle Scholar
- Black WC IV, Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A. 1994;91:10034–8. DOIPubMedGoogle Scholar
- McGill SK, Hashash JG, Platts-Mills TA. AGA clinical practice update on alpha-gal syndrome for the GI clinician [commentary]. Clin Gastroenterol Hepatol. 2023;21:891–6. DOIPubMedGoogle Scholar
- Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16:667–77. DOIPubMedGoogle Scholar
- Keirans JE, Lacombe EH. First records of Amblyomma americanum, Ixodes (Ixodes) dentatus, and Ixodes (Ceratixodes) uriae (Acari: Ixodidae) from Maine. J Parasitol. 1998;84:629–31. DOIPubMedGoogle Scholar
- The University of Maine Cooperative Extension. UMaine Tick Surveillance Program annual report 2022. Orono (ME): The University; 2022.
- The University of Maine Cooperative Extension. UMaine Tick Surveillance Program annual report 2023. Orono (ME): The University; 2023.
- Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, et al. Galactose-α-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013;131:e1545–52. DOIPubMedGoogle Scholar
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–93.e6. DOIPubMedGoogle Scholar
- Kersh GJ, Salzer J, Jones ES, Binder AM, Armstrong PA, Choudhary SK, et al. Tick bite as a risk factor for alpha-gal-specific immunoglobulin E antibodies and development of alpha-gal syndrome. Ann Allergy Asthma Immunol. 2023;130:472–8. DOIPubMedGoogle Scholar
- Wilson JM, Keshavarz B, James HR, Retterer MKC, Schuyler AJ, Knoedler A, et al. α-Gal specific-IgE prevalence and levels in Ecuador and Kenya: Relation to diet, parasites, and IgG4. J Allergy Clin Immunol. 2021;147:1393–1401.e7. DOIPubMedGoogle Scholar
- Murangi T, Prakash P, Moreira BP, Basera W, Botha M, Cunningham S, et al. Ascaris lumbricoides and ticks associated with sensitization to galactose α1,3-galactose and elicitation of the alpha-gal syndrome. J Allergy Clin Immunol. 2022;149:698–707.e3. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: March 19, 2025
1These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 4—April 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Johanna S. Salzer, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H24-12, Atlanta, GA 30329-4018, USA
Top