Safety and Immunogenicity of Poultry Vaccine for Protecting Critically Endangered Avian Species against Highly Pathogenic Avian Influenza Virus, United States
Todd E. Katzner

, Ashleigh V. Blackford, Mary Donahue, Samantha E.J. Gibbs, Julianna Lenoch, Michael Martin, Tonie E. Rocke, J. Jeffrey Root, Darrel Styles, Sunny Cooper, Kristin Dean, Zachary Dvornicky-Raymond, Dominique Keller, Carlos Sanchez, Brett Dunlap, Thomas Grier, Michael P. Jones, Gregory Nitzel, Erin Patrick, Maureen Purcell, Aaron J. Specht, and David L. Suarez
Author affiliation: US Geological Survey, Forest and Rangeland Ecosystem Science Center, Boise, Idaho, USA (T.E. Katzner, M. Purcell); US Fish and Wildlife Service, California Condor Recovery Program, Vero Beach, Florida, USA (A.V. Blackford); US Department of Agriculture, Animal and Plant Health Inspection Service (APHIS) Veterinary Services, Minneapolis, Minnesota, USA (M. Donahue); US Fish and Wildlife Service, National Wildlife Refuge System, Wildlife Health Office, Chiefland, Florida, USA (S.E.J. Gibbs); US Department of Agriculture, APHIS Wildlife Services, National Wildlife Disease Program, Fort Collins, Colorado, USA (J. Lenoch); North Carolina Department of Agriculture and Consumer Services, Raleigh, North Carolina, USA (M. Martin); US Geological Survey, National Wildlife Health Center, Madison, Wisconsin, USA (T.E. Rocke); US Department of Agriculture, APHIS Wildlife Services, National Wildlife Research Center, Fort Collins (J.J. Root); US Department of Agriculture, APHIS Veterinary Services, Riverdale, Maryland, USA (D. Styles); Carolina Raptor Center, Huntersville, North Carolina, USA (S. Cooper, K. Dean); San Diego Zoo Wildlife Alliance, San Diego, California, USA (Z. Dvornicky-Raymond); Los Angeles Zoo and Botanical Garden, Los Angeles, California, USA (D. Keller); Oregon Zoo, Portland, Oregon, USA (C. Sanchez); US Department of Agriculture, APHIS Wildlife Services, Madison, Tennessee, USA (B. Dunlap); Purdue University School of Health Sciences, West Lafayette, Indiana, USA (T. Grier, A.J. Specht); American Eagle Foundation, Kodak, Tennessee, USA (M.P. Jones); Zoetis, Inc., Kalamazoo, Michigan, USA (G. Nitzel); US Department of Agriculture, APHIS Wildlife Services, Knoxville, Tennessee, USA (E. Patrick); US Department of Agriculture, Southeast Poultry Research Laboratory, Agricultural Research Service, Athens, Georgia, USA (D.L. Suarez)
Main Article
Figure 2
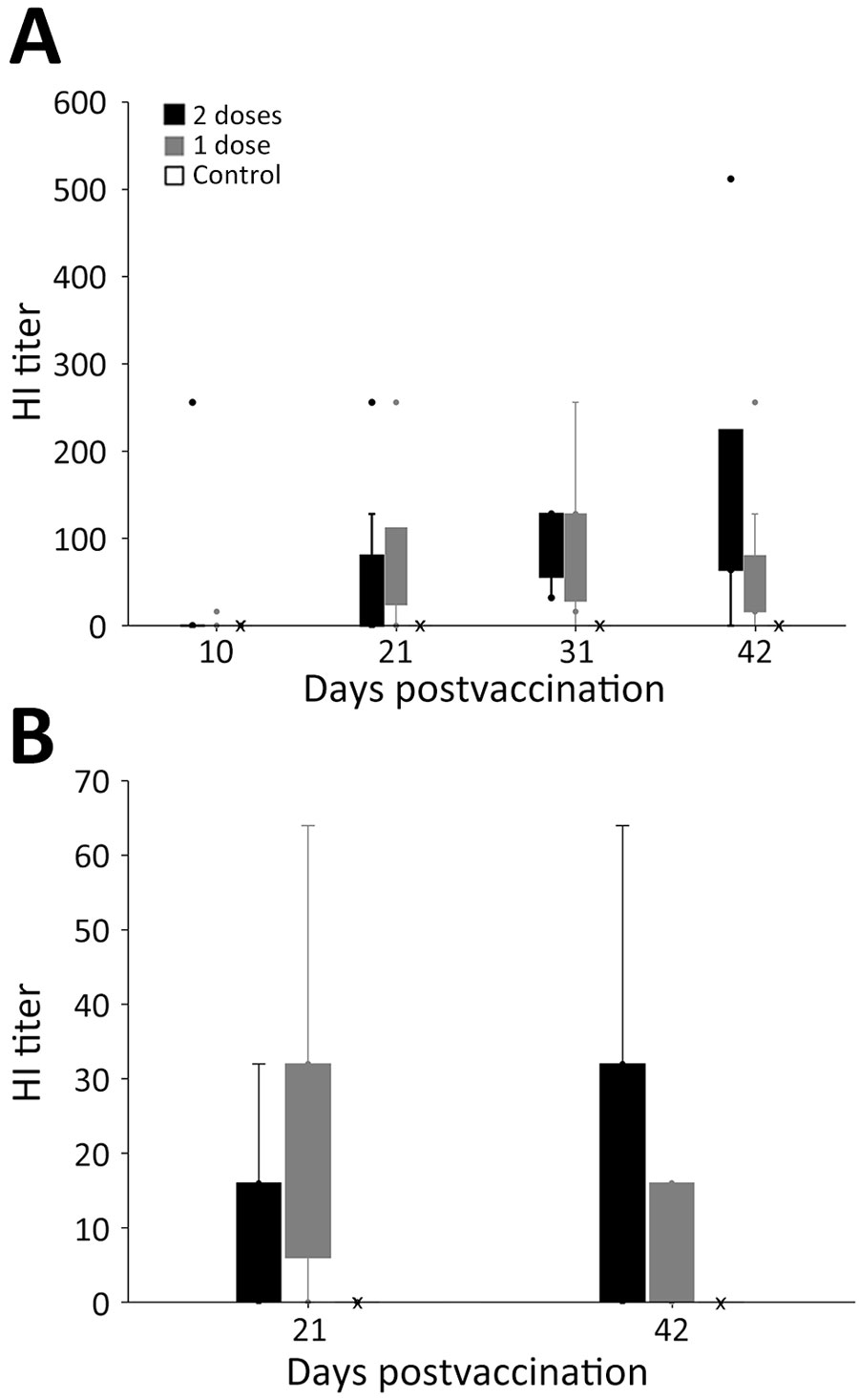
Figure 2. Hemagglutination inhibition (HI) titers in a study of safety and immunogenicity of poultry vaccine for protecting critically endangered avian species from highly pathogenic avian influenza virus, United States. A) Titers for 28 black vultures (Coragryps atratus); B) titers for 25 California condors (Gymnogyps californianus). Birds were included in 1 of 3 highly pathogenic avian influenza vaccine trial groups comprised of 10 birds given two 0.5-mL vaccinations at days 0 and 21, another 10 birds given a single 1-mL vaccination at day 0, and 8 vultures and 5 condors that were unvaccinated negative controls. Vaccinated animals were given a 1057.R1 serial 590088 avian influenza vaccine, H5N1 subtype, reverse genetics-derived, inactivated vaccine (see main text for details on the vaccine). For vultures, postvaccination blood draws were conducted at 10, 21, 31, and 42 days after first vaccination; for condors, blood draws were on days 21 and 42. Box tops and bottoms show quartiles, whiskers are 95% CI, dots outliers; X indicates 0 values for control groups.
Main Article
Page created: April 03, 2025
Page updated: May 27, 2025
Page reviewed: May 27, 2025
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
