Volume 31, Number 6—June 2025
Research Letter
Rapid Subcutaneous Migration of Dirofilaria repens Nematode in Facial Tissue, Italy
Abstract
We report a Dirofilaria repens nematode infection in a woman in Italy who sought care for a fast-creeping lesion within her subcutaneous facial tissue. Dirofilariosis should be included in differential diagnosis of subcutaneous nodules or creeping lesions. This case highlights the need for controlling canine dirofilarioses to mitigate zoonotic risk.
Dirofilarioses are mosquitoborne zoonotic diseases caused by filarial nematodes, which affect domestic and wild carnivores in tropical, subtropical, and temperate areas worldwide (1). Humans act as dead-end hosts because the third stage larvae, which are transmitted by mosquitoes during the blood meal, do not usually reach sexual maturity. Human cases of dirofilariosis have been documented worldwide; Dirofilaria immitis and D. repens nematodes have been reported from both the New and Old World (2). In humans, subcutaneous lesions caused by D. repens nematode infection can occur in several anatomic areas (e.g., forehead, arms, and periorbital and perioral areas) and, rarely, in deeper tissues (e.g., lymph nodes, lungs, muscles, and dura) (3). In addition, pulmonary localization of Dirofilaria spp. is characterized by the presence of solitary, well-circumscribed, noncalcified peripheral subpleural pulmonary nodules (coin lesions), which mimic lung cancer (4). In the Mediterranean Basin, ideal climatic conditions for the development of mosquito vectors, as well as the high prevalence of microfilaremic dogs, are risk factors for human infections, as observed in areas highly endemic for canine dirofilariosis, such as southern Italy (5,6). Specifically, in Europe, D. repens is considered an emerging pathogen, because it presents an expanding distribution linked to an increasing number of human cases (4). We report a case of human dirofilariosis in a woman in her forties, living in Rome, Italy, with 3 cats as pets.
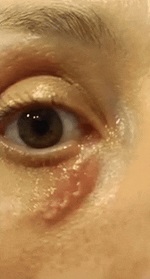
The patient first underwent ophthalmologic consultation because of visual impairment; her condition was initially misdiagnosed as an allergic reaction of the right upper eyelid. Five days later, the patient was referred to the National Institute for Infectious Diseases Lazzaro Spallanzani after she reported a worm-like organism creeping within the subcutaneous tissue of the right lower periorbital region (Video; Appendix Figure). No other clinical signs (e.g., dermo-epidermal eruptive patches) were recorded besides retroauricular lymphadenomegaly and low-grade fever (up to 38.0°C). The patient had not recently traveled abroad.
After signing informed consent, the patient was hospitalized but surgery was not performed because the suspected parasite (likely Dirofilaria spp.) had migrated to the right parietal area of the head (i.e., the subcutaneous tissue at the parietal bone of the skull), preventing its removal. During her 5-day hospitalization, the patient was in good clinical condition; hematological and serologic biochemical parameters were within reference ranges; eosinophilia was not present. Infections by Strongyloides stercoralis and zoonotic filarial worms (i.e., Brugia spp., Wuchereria bancrofti, Mansonella spp., and Oncocherca spp.) were excluded by serologic assays (i.e., commercial ELISA kits). Chest radiography was performed to exclude the presence of coin lesions typical of Dirofilaria spp. infection.
We tested a serum sample at the Department of Public Health and Infectious Diseases Sapienza, University of Rome, to assess exposure to Dirofilaria spp. by using an in-house ELISA based on somatic antigens of adult D. repens (6,7), which yielded positive results (i.e., optical density 1.56; optical density cut off 1.03 for D. repens). The woman was discharged from the hospital with the recommendation to return on observation of parasite reemergence to the facial subcutaneous tissue.
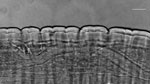
Two weeks later, the nematode migrated in the frontal area, and a surgical excision was performed under local anesthesia. The specimen was shipped to the University of Bari (Italy) for further morphological and molecular analysis. The fragmented nematode was morphologically identified as a mature female, cylindrical, ≈2.96 cm in length, and 0.480 mm thick (Figure 1). Microscopic analysis revealed a thick laminated cuticle with characteristic longitudinal ridges and cross-striations (Figure 1), leading to the identification of the parasite as D. repens (8).
Genomic DNA was extracted from the nematode and tested by conventional PCR targeting cox1 gene (9) to obtain a reference sequence. BLAST (https://blast.ncbi.nlm.nih.gov) analysis revealed 100% nucleotide identity with reference sequence of D. repens in the GenBank database (accession no. MW675692), which further confirmed by phylogenetic analyses (Figure 2). At the 5-month follow-up, the patient’s only residual symptom was a persisting uncomfortable feeling, likely associated with parasite migration. We also performed specific tests to detect Dirofilaria spp. on her pets, yielding negative results.
The increasing incidence of human cases of dirofilariosis in Europe (10) underscores the need for including this emerging zoonotic disease in the differential diagnosis of pulmonary or subcutaneous nodules in absence of eosinophilia. The rapid migration of the nematode in this case was unusual, highlighting the variability of clinical signs in patients infected by D. repens nematodes, which range from stationary nodules to fast-migrating lesions in subcutaneous tissues. Human dirofilariosis is typically an abortive infection because humans are accidental hosts, and microfilaremia is absent (1). Consequently, traditional diagnostic methods applied in veterinary medicine (e.g., Knott’s test) are unsuitable. Definitive diagnosis in human patients is challenging, and often only achievable after surgical removal of the parasite.
In summary, we identified D. repens nematode infection in a woman with a creeping lesion in her subcutaneous facial tissue. This case highlights the need for a One Health approach in implementing vector control strategies and regular monitoring of reservoir hosts in endemic areas to mitigate the risk for human D. repens infection.
Dr. Carbonara is a research fellow at the Department of Veterinary Medicine, University of Bari. Her main research interest is in vectorborne pathogens of zoonotic concern in both animals and humans.
Acknowledgments
We are grateful to the patient involved in the study and to Livia Perles for assistance with the phylogenetic analysis.
No funding has been employed for the present work. D.O., J.A.M.R., and R.I. were partially supported by European Union funding within the NextGeneration EU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (project no. PE00000007, INF-ACT).
References
- Perles L, Dantas-Torres F, Krücken J, Morchón R, Walochnik J, Otranto D. Zoonotic dirofilariases: one, no one, or more than one parasite. Trends Parasitol. 2024;40:257–70. DOIPubMedGoogle Scholar
- Dantas-Torres F, Otranto D. Dirofilariosis in the Americas: a more virulent Dirofilaria immitis? Parasit Vectors. 2013;6:288. DOIPubMedGoogle Scholar
- Grapatsas K, Kayser G, Passlick B, Wiesemann S. Pulmonary coin lesion mimicking lung cancer reveals an unexpected finding: Dirofilaria immitis. J Thorac Dis. 2018;10:3879–82. DOIPubMedGoogle Scholar
- Capelli G, Genchi C, Baneth G, Bourdeau P, Brianti E, Cardoso L, et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit Vectors. 2018;11:663. DOIPubMedGoogle Scholar
- Otranto D, Brianti E, Gaglio G, Dantas-Torres F, Azzaro S, Giannetto S. Human ocular infection with Dirofilaria repens (Railliet and Henry, 1911) in an area endemic for canine dirofilariasis. Am J Trop Med Hyg. 2011;84:1002–4. DOIPubMedGoogle Scholar
- Mendoza-Roldan JA, Gabrielli S, Cascio A, Manoj RRS, Bezerra-Santos MA, Benelli G, et al. Zoonotic Dirofilaria immitis and Dirofilaria repens infection in humans and an integrative approach to the diagnosis. Acta Trop. 2021;223:
106083 . DOIPubMedGoogle Scholar - Potters I, Vanfraechem G, Bottieau E. Dirofilaria repens nematode infection with microfilaremia in traveler returning to Belgium from Senegal. Emerg Infect Dis. 2018;24:1761–3. DOIPubMedGoogle Scholar
- Otranto D, Eberhard ML. Zoonotic helminths affecting the human eye. Parasit Vectors. 2011;4:41. DOIPubMedGoogle Scholar
- Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93–103. DOIPubMedGoogle Scholar
- Simón F, Diosdado A, Siles-Lucas M, Kartashev V, González-Miguel J. Human dirofilariosis in the 21st century: A scoping review of clinical cases reported in the literature. Transbound Emerg Dis. 2022;69:2424–39. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: May 20, 2025
Table of Contents – Volume 31, Number 6—June 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Domenico Otranto, University of Bari Aldo Moro, Str. prov. per Casamassima km 3, 70010 Valenzano (Bari), Italy
Top