Volume 31, Number 7—July 2025
Research Letter
Fatal Acute Hypoxemic Respiratory Failure Caused by Burkholderia thailandensis, China
Abstract
We report on a patient in China with no underlying illnesses who died of Burkholderia thailandensis infection despite timely treatment. This case challenges the perception that B. thailandensis is nonlethal or has low virulence. Increased clinical awareness and prompt diagnosis are essential for managing B. thailandensis infections and preventing fatal outcomes.
Burkholderia thailandensis is commonly regarded as nonpathogenic, whereas B. pseudomallei is recognized as the most clinically relevant species known to cause melioidosis (1). We describe a rare case of fatal acute hypoxemic respiratory failure and septic shock caused by B. thailandensis in a previously healthy person from Hainan Province, China. The Institutional Review Board at Hainan General Hospital approved the study protocol.
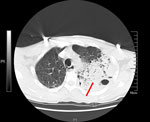
A 58-year-old male rice farmer from the central region of Hainan Province was admitted to an emergency department on May 23, 2019. He exhibited an unexplained onset of cough, sputum production, chest tightness, and shortness of breath that had persisted for 8 days. He did not smoke, have a previous family history of those respiratory conditions, or have immune deficiencies. His vital signs and laboratory test results were recorded at admission (Appendix Table 1). His acute physiology and chronic health evaluation II score was 24 and sequential organ failure assessment score was 13 after 1 day of hospitalization. A chest computed tomography scan (Figure) revealed a large high-density shadow in the left lung field and a small effusion in the left pleural cavity, indicating a substantial infection in the left lung. Tigecycline was administered for infection control.
Bronchoalveolar lavage fluid was submitted for bacterial culture on May 24, 2019. After 48 hours of incubation, colonies consisted of short gram-negative bacilli (Appendix Figure 1). We performed matrix-assisted laser desorption/ionization time-of-flight mass spectrometry profiling (Appendix Figure 2), 16S rRNA-based phylogenetic tree analysis (Appendix Figure 3, panel A), and biochemical identification (Appendix Figure 4). We identified the isolate as B. thailandensis strain HNBT001.
Whole-genome sequencing using Illumina (https://www.illumina.com) and Pacific Biosciences (https://www.pacb.com) pipelines revealed the B. thailandensis strain consisted of 2 circular sequences: a 3,929,948-bp chromosome and a 2,858,975-bp chromosome (GenBank accession no. GCA_048688115.1), having an average G/C nucleotide content of 67.53%. We used values of digital DNA–DNA hybridization (dDDH) and average nucleotide identity (ANI) to compare the isolated strain with representative genomes of Burkholderia spp. in the National Center for Biotechnology Information RefSeq database (https://www.ncbi.nlm.nih.gov/refseq; accessed on June 6, 2024). Using the Type Genome Server (2), we found pairwise comparison with a B. thailandensis reference genome (assembly no. GCF_001718635.1) indicated the genomes of the strain in our study had a dDDH formula d4 value of 89% and an ANI value of 98.9%; both values exceeded the thresholds for prokaryotic species delineation (70% for dDDH) (3) and bacterial species differentiation (96% for ANI) (Appendix Figure 5) (4). The HNBT001 strain was classified as sequence type 80, previously identified in both non–human-associated (E264) and human-associated (FDAARGOS 240 and 2022Dzh) strains (Appendix Figure 3, panel B).
On the basis of antimicrobial susceptibility test results (Appendix Table 2), we replaced tigecycline with imipenem for infection treatment. The patient’s symptoms lessened after 2 days of imipenem therapy. However, despite a transient improvement in symptoms, his blood oxygenation gradually deteriorated while receiving invasive mechanical ventilation support, leading to multiorgan dysfunction, manifesting as scattered low-density lesions of the liver, splenomegaly, persistent thrombocytopenia, and acute renal failure characterized by substantially elevated serum creatinine levels. In addition, on day 6 of hospitalization, peripheral edema developed. After progressive clinical deterioration, the patient elected to withdraw from further treatment and was discharged after an 8-day hospital admission. During hospitalization, he was treated with imipenem for 5 days. The patient died 3 days after discharge.
B. thailandensis is generally considered nonpathogenic and is commonly found in tropical and subtropical environments, particularly in humid soil and water (5). In this case, the patient was a farmer who had early clinical manifestations of cough, phlegm, chest tightness, and shortness of breath and was likely exposed to B. thailandensis through contact with contaminated soil or water. However, unlike most reported cases, this patient had no previous underlying health conditions.
This case occurred in Hainan Province, located >1,400 km from regions where 2 other cases have been documented in China (6,7). However, medical experts with extensive experience believed insufficient evidence existed to identify B. thailandensis in the first case (6,8). Through 16S rRNA gene analysis (Appendix Figure 3) and taxonomic comparisons in GenBank (accession no. GCA_011578485.1), we classified the strain in this case as B. thailandensis. In the previous case involving wound infection (7), colonization by nonvirulent B. thailandensis could not be definitively excluded. In our case, despite the identification of multiple potential virulence genes in the HNBT001 strain (Appendix Table 3), exact pathogenic mechanisms remain to be elucidated through further research. Because of the high potential for B. thailandensis to be misidentified as B. pseudomallei (9), the prevalence and public health impact of B. thailandensis might be considerably underestimated.
In conclusion, contrary to the previously held opinion that B. thailandensis is nonpathogenic or of low virulence, we show it can cause severe infections even in immunocompetent patients and potentially fatal outcomes in otherwise healthy persons. We strongly advise medical personnel to place greater emphasis on strengthening biosafety precautions during both laboratory work and clinical treatment involving this underestimated pathogen.
Ms. Zhang is a research scientist in clinical microbiology at Hainan General Hospital, Haikou, China. Her research focuses on the study of pathogenic microorganisms within medical institutions to enhance knowledge of infectious diseases and their effects on public health.
Acknowledgments
The patient’s family provided written informed consent encompassing the disclosure of clinical particulars, identifying images, and other relevant data.
This work was supported by the Hainan Province Science and Technology Special Fund (fund no. ZDYF2022SHFZ050), National Natural Science Foundation of China (grant nos. 82370018 and 82000011), National Natural Science Fund Cultivating 530 Project of Hainan General Hospital, and Hainan Clinical Research Center.
Contributions: D.K., H.W., and Q.X. conceived and designed the experiments. P.Z., S.C., X.D., X,W., and D.K. performed the experiments. D.K., W.L., and Y.C. analyzed and interpreted the data. H.W. and Q.X. provided reagents, materials, and analysis data. P.Z. and D.K. wrote the paper.
References
- Yu Y, Kim HS, Chua HH, Lin CH, Sim SH, Lin D, et al. Genomic patterns of pathogen evolution revealed by comparison of Burkholderia pseudomallei, the causative agent of melioidosis, to avirulent Burkholderia thailandensis. BMC Microbiol. 2006;6:46. DOIPubMedGoogle Scholar
- Meier-Kolthoff JP, Göker M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun. 2019;10:2182. DOIPubMedGoogle Scholar
- Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. DOIPubMedGoogle Scholar
- Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009;106:19126–31. DOIPubMedGoogle Scholar
- Hall CM, Stone NE, Martz M, Hutton SM, Santana-Propper E, Versluis L, et al. Burkholderia thailandensis isolated from the environment, United States. Emerg Infect Dis. 2023;29:618–21. DOIPubMedGoogle Scholar
- Chang K, Luo J, Xu H, Li M, Zhang F, Li J, et al. Human infection with Burkholderia thailandensis, China, 2013. Emerg Infect Dis. 2017;23:1416–8. DOIPubMedGoogle Scholar
- Li J, Tan J, Xiong X, Zhong Q, Lu W. Burkholderia thailandensis isolated from infected wound, southwest China, 2022. Emerg Infect Dis. 2024;30:1055–7. DOIPubMedGoogle Scholar
- Dance DAB, Sarovich D, Price EP, Limmathurotsakul D, Currie BJ. Human infection with Burkholderia thailandensis, China, 2013. Emerg Infect Dis. 2018;24:953–4. DOIPubMedGoogle Scholar
- Watthanaworawit W, Roberts T, Hopkins J, Gassiep I, Norton R, Robinson MT, et al. A multi-country study using MALDI-TOF mass spectrometry for rapid identification of Burkholderia pseudomallei. BMC Microbiol. 2021;21:213. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: May 21, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 7—July 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Addresses for correspondence: Hua Wu, Department of Laboratory Medicine, Hainan General Hospital, No.19, Xiuhua Road, Xiuying District, Haikou 570311, China
Top