Volume 31, Number 8—August 2025
Dispatch
Microsporidial Keratoconjunctivitis Caused by Vittaforma corneae, Sea of Galilee, Israel, 2022–2024
Cite This Article
Citation for Media
Abstract
We describe a multiannual outbreak of keratoconjunctivitis caused by the microsporidium Vittaforma corneae in the Sea of Galilee, Israel. Patients had multifocal punctate corneal infiltrates and reduced visual acuity, confirmed by locally-developed pathogen-specific real-time PCR. Topical chlorhexidine, rather than traditional antimicrobial drugs, proved an effective and safe primary treatment.
Microsporidial keratitis, caused by spore-forming unicellular parasites now classified as fungi, has previously been recognized as a severe yet uncommon ocular infection, often associated with outbreaks worldwide (1,2). Vittaforma corneae (previously known as Nosema corneum) was first identified in a child from Sri Lanka in 1973 (3) and has emerged as a keratoconjunctivitis pathogen, especially in water-related outbreaks. V. corneae is characterized by small spores (3–4-μm long and 1–1.5μm wide), has unique ultrastructural features, and exhibits a specific affinity for ocular tissues.
The natural reservoir of V. corneae remains unknown, yet it has been detected in various mammals and invertebrates, suggesting a broad host range. Of note, humans are not considered natural hosts of V. corneae. Environmental sources, particularly aquatic environments, are believed to play a crucial role in V. corneae transmission and persistence. Studies have identified V. corneae spores in both fresh and marine water samples, indicating its ability to survive in diverse aquatic settings (1,2).
In this article, we describe a multiannual outbreak of V. corneae keratoconjunctivitis associated with exposure to the Sea of Galilee in northern Israel. This outbreak is noteworthy for its prolonged duration and specific geographic association, which has not been previously reported for V. corneae infections. Our PCR-confirmed cases of V. corneae keratoconjunctivitis represent dozens of instances nationwide. This study was approved by the Meir Medical Center Internal Review Board (approval no. 0001-24-MMC).
During 2022–2024, we detected 12 PCR-confirmed patients, 6–51 years of age (median age 15.3 years; mean ± SD age 22.29 ± 19.76 years); 5 were female and 7 were male. All reported recent exposure to the Sea of Galilee before symptom onset, with a median duration of 14 days (range 10–18 days) and an average of 13.75 days (SD ± 2.33 days) between exposure and symptoms. Similar to other nationwide reported cases, no other epidemiologic links were identified (Appendix).
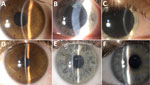
Patients reported eye symptoms of redness, watering, irritation, and a foreign-body sensation. Visual acuity when seeking care averaged 0.60 ± 0.34 decimal (normal vision >1.0 decimal). Slit-lamp examination revealed coarse, multifocal, punctate epithelial lesions (<1 mm) on the cornea, often accompanied by nonpurulent conjunctivitis with a mixed follicular-papillary reaction (Figure). Those findings were uniform across the patient cohort and consistent with previous descriptions of V. corneae infections.
During the early months of the outbreak, the causative organism was unidentified. The ophthalmologic findings matched those of previously reported microsporidial infections and were supported by microscopy of corneal scraping specimens. We found numerous oval spores 3–5µm long and 1–2µm wide (4; https://www.cdc.gov/dpdx/microsporidiosis/index.html) by using fluorescent calcofluor staining. We used a pan-microsporidial PCR targeting the small subunit rRNA of most microsporidia (ss18f, 5′-caccaggttgattctgcc-3′; ss1492r, 5′-ggttaccttgttacgactt-3′) (5), but it failed to identify any specific pathogen, likely because of limited corneal scraping material and other technical limitations, such as primer mismatches with the target species. Next, we performed a shotgun metagenomic sequencing on pooled corneal scrapings by using a Nextera XT library and Illumina MiSeq platform (Illumina, https://www.illumina.com), yielding >7 million reads. Despite a predominance of host-derived sequences, species-specific reads mapping to the V. corneae small subunit rRNA gene verified the presence of V. corneae. To confirm this result, we developed a SYBR Green–based real-time PCR assay for V. corneae, following the methodology outlined previously (6). The assay used the primer sequences corn-F 5′-ctaccaagacagtgacggttga-3′ and corn-R 5′-ggcatcttttactgctggaact-3′. We conducted Sanger sequencing on the amplicons, yielding 100% coverage and >95% identity with V. corneae.
Our key finding was the efficacy of topical chlorhexidine as a first-line treatment after corneal debridement, which was therapeutic and diagnostic. All patients were treated with 0.02% chlorhexidine 2–3 times daily, demonstrating excellent tolerability and outcomes. This regimen marks a shift from the combination therapies (sometimes including systemic drugs) typically used for microsporidial keratitis (7,8).
In contrast, only 3 patients in our study received either topical moxifloxacin or topical voriconazole (2–3 times daily), and 3 were treated with short courses of adjunctive topical steroids during follow-up (Appendix Table 1). Of note, no patients required hospitalization or additional interventions beyond topical therapy. Chlorhexidine was well tolerated, and no major corneal scarring or reported ocular discomfort related to its use was reported. Those findings support the potential of a simplified, topical-only approach to treatment.
Visual acuity improved in most patients, and the mean at the last follow-up (average 6.1 ± 4.2 months) reached 0.87 ± 0.21 decimal visual acuity. Although not statistically significant (p = 0.1) because of the small sample size, the improvement in visual acuity does indicate that topical chlorhexidine is effective in preserving visual function and preventing disease progression.
In this article, we present a large outbreak of PCR-confirmed V. corneae keratoconjunctivitis associated with a single freshwater body, the Sea of Galilee, which has not previously been linked to a microsporidial outbreak. The multiannual nature of this outbreak, spanning >3 consecutive years, suggests the presence of a persistent environmental reservoir of V. corneae in this ecosystem, potentially influenced by unique ecologic conditions or anthropogenic factors. This outbreak is of public health interest given the widespread recreational use of the Sea of Galilee, which is the only major freshwater lake in Israel.
Our findings underscore the effectiveness and safety of topical chlorhexidine as a treatment for V. corneae keratoconjunctivitis. This simple, cost-effective regimen achieved favorable outcomes without the need for complex multidrug therapies or hospitalization. Although chlorhexidine avoids unnecessary exposure to systemic antimicrobial drugs and offers broad-spectrum coverage, this regimen may be insufficient in more complicated cases, such as contact lens–related infections involving Pseudomonas spp., other more complex pathogens, or immunocompromised hosts. Corneal scarring did not develop in any of our patients, suggesting chlorhexidine can preserve corneal integrity.
Early and accurate diagnosis was essential for guiding appropriate treatment. Because of the rarity of microsporidial infections in Israel, a species-specific real-time PCR enabled rapid, reliable detection of V. corneae from limited ocular samples. PCR proved especially useful in settings where microsporidial keratitis was not routinely suspected, enabling timely therapy.
The first limitation of this study is that, apart from 1 patient with untreated stable sarcoidosis, all patients were immunocompetent, limiting applicability to immunocompromised populations who may require more intensive treatment and prolonged follow-up and whose keratitis could be associated with systemic infection (9). Finally, the restriction of the outbreak to a single, ecologically unique body of water, the Sea of Galilee, limits the broader generalizability of these findings.
Further research is needed to clarify the ecologic and microbiological factors contributing to the persistence of V. corneae in the Sea of Galilee and to assess the potential for similar outbreaks elsewhere. This event also emphasizes the need for public health measures, including environmental monitoring and preventive recommendations, highlighting an emerging pattern in waterborne microsporidial infections, and the need for increased awareness among clinicians and microbiologists. PCR was essential for rapid and accurate pathogen identification, and the success of chlorhexidine 0.02% as a primary therapy offers a promising, simplified approach for managing such infections.
Dr. Friehmann is the director of the Cornea Service at Meir Medical Center, Kfar Sava. His research interests include corneal infections, trauma, and transplantation.
Acknowledgment
We thank the Genetic Laboratory and the Bioinformatics Unit at the Israel Institute for Biological Research for their invaluable support in performing the metagenomic sequencing and analysis.
References
- Sabhapandit S, Murthy SI, Garg P, Korwar V, Vemuganti GK, Sharma S. Microsporidial stromal keratitis: clinical features, unique diagnostic criteria, and treatment outcomes in a large case series. Cornea. 2016;35:1569–74. DOIPubMedGoogle Scholar
- Moshirfar M, Somani SN, Shmunes KM, Espandar L, Gokhale NS, Ronquillo YC, et al. A narrative review of microsporidial infections of the cornea. Ophthalmol Ther. 2020;9:265–78. DOIPubMedGoogle Scholar
- Ashton N, Wirasinha PA. Encephalitozoonosis (nosematosis) of the cornea. Br J Ophthalmol. 1973;57:669–74. DOIPubMedGoogle Scholar
- Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol. 1995;33:3138–45. DOIPubMedGoogle Scholar
- Ghosh K, Weiss LM. Molecular diagnostic tests for microsporidia. Interdiscip Perspect Infect Dis. 2009;2009:
926521 . DOIPubMedGoogle Scholar - Jayahar Bharathi M, Murugan N, Ramesh Kumar G, Ramakrishnan R, Anitha V, Ramesh S. Vittaforma corneae keratitis in southern India: role of a novel duplex PCR. J Med Microbiol. 2013;62:553–9. DOIPubMedGoogle Scholar
- Ramatchandirane B, A MK, Marimuthu Y, Nicodemus DS, Yarra MC. A MK, Marimuthu Y, Nicodemus DS, Yarra MC. Successful treatment of microsporidial keratoconjunctivitis (MKC) with a combination of topical voriconazole 1% and gatifloxacin 0.5%: a large case series of 29 patients. Cureus. 2023;15:
e49247 .PubMedGoogle Scholar - Coca M, Kim J, Shenoy S, Chévez-Barrios P, Kapur M. Microsporidial stromal keratitis: successful treatment with topical voriconazole and oral itraconazole. Cureus. 2016;8:
e934 . DOIPubMedGoogle Scholar - Yeh TC, Kuo YS, Wang LC, Tai TY, Lin PY. Chlorhexidine in the treatment of microsporidial stromal keratitis and the effect of host immunity: A case series and literature review. J Chin Med Assoc. 2022;85:532–6. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: July 02, 2025
Table of Contents – Volume 31, Number 8—August 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sharon Amit, Sheba Medical Center, Derech Sheba 2, Ramat-Gan, 5266202, Israel
Top