Volume 31, Number 9—September 2025
CME ACTIVITY - Research
Rickettsioses as Underrecognized Cause of Hospitalization for Febrile Illness, Uganda
Figure 1
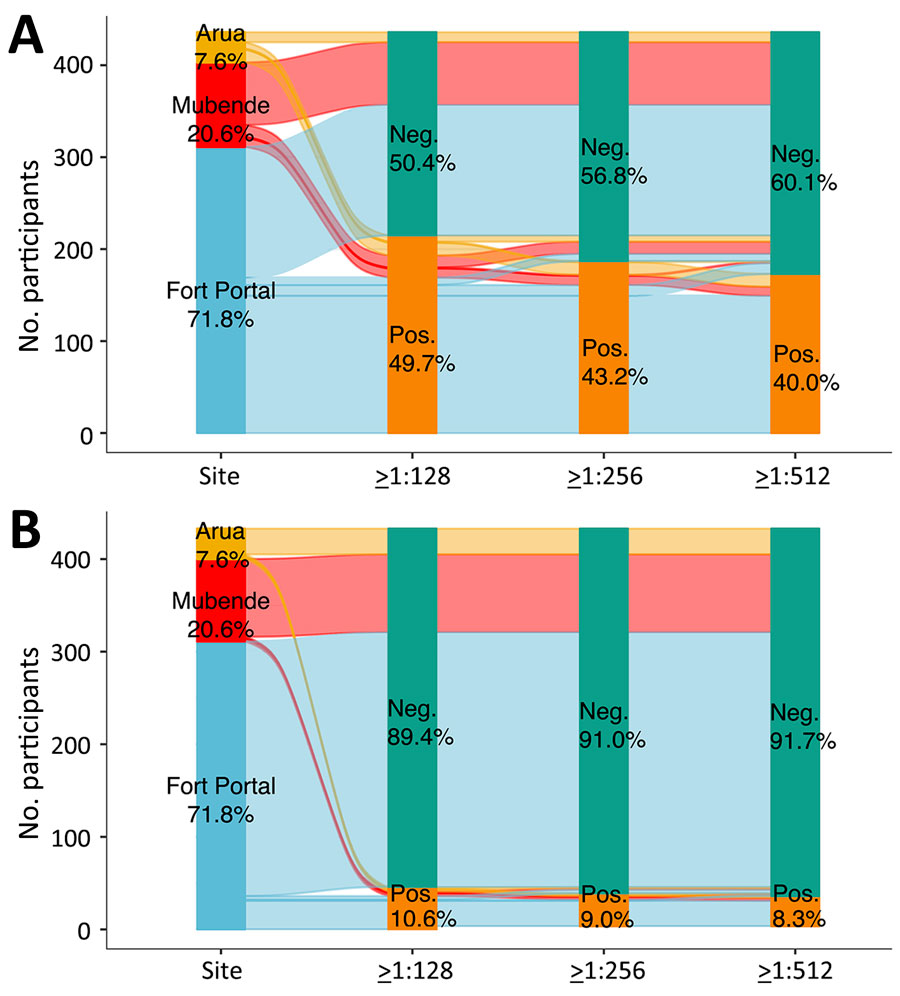
Figure 1. Alluvial plots of baseline acute serum samples from study of rickettsioses as an underrecognized cause of hospitalization for febrile illness, Uganda. A) Spotted fever group rickettsiae; B) typhus group rickettsiae. Immunofluorescence assay IgG seroprevalence is shown for different sites and different titer cutoffs. Participant samples were from referral hospital clinical study sites in Arua (in yellow; 7.6% of participants), Mubende (in red, 20.6% of participants), and in Fort Portal (in blue, 71.8%). Distribution of the colored lines across the graph shows a comparison of positive or negative samples among the sites. Green is the total percentage of negative samples. Orange is the total percentage of positive samples. Neg, negative; pos, positive.
1Members are listed at the end of this article.