Volume 4, Number 2—June 1998
Perspective
Wild Primate Populations in Emerging Infectious Disease Research: The Missing Link?
Cite This Article
Citation for Media
Abstract
Wild primate populations, an unexplored source of information regarding emerging infectious disease, may hold valuable clues to the origins and evolution of some important pathogens. Primates can act as reservoirs for human pathogens. As members of biologically diverse habitats, they serve as sentinels for surveillance of emerging pathogens and provide models for basic research on natural transmission dynamics. Since emerging infectious diseases also pose serious threats to endangered and threatened primate species, studies of these diseases in primate populations can benefit conservation efforts and may provide the missing link between laboratory studies and the well-recognized needs of early disease detection, identification, and surveillance.
Infectious diseases respect no species or geographic boundaries. For a parasite, closely related hosts offer new environments in which infection, maintenance, replication, and transmission remain possible. The anthropoid primates (which include humans) and to a lesser degree simian primates share broadly similar physiologic and genetic characteristics and thus susceptibility to many viruses, bacteria, fungi, protozoa, helminths, and ectoparasites (1) that have the potential to cross primate-species boundaries (2).
Similarities in pathogen susceptibility have made nonhuman primates ideal laboratory models. During the 20th century, laboratory research on captive primates has elucidated the life cycle and pathogenesis of many infectious agents and facilitated drug and vaccine development. Nevertheless, the ecology of infectious agents found in wild populations of primates has only recently been addressed. Just as captive primates have proved invaluable for research at the level of the organism, wild populations can provide the opportunity to study infectious disease phenomena at the population and ecosystem levels. Research at these levels addresses such pressing questions as the origin(s) of pathogens, determinants of pathogen emergence, and factors influencing maintenance of pathogens in animal reservoirs.
During the past two decades unknown human diseases, including AIDS, Ebola fever, hantavirus infection, and dengue hemorrhagic fever, have emerged from enzootic foci. The emergence of these and other diseases has been linked to the interface of tropical forest communities with high levels of biodiversity and agricultural communities with relative genetic homogeneity and high population densities of humans, domestic animals, and crops. This interface poses a high risk for the emergence of novel disease (3-5).
Since most nonhuman primates live in tropical forest habitats, most interactions between humans and wild nonhuman primates occur in this high-risk interface, which has recently increased because of expanded ecotourism and forest encroachment. These interactions can lead to pathogen exchange through various routes of transmission (Table). Arthropod vectors, shared water, and hunting of wild animals have facilitated pathogen exchange and may have played an important role in pathogen transfers since ancient times. In the recent past, laboratory research has led to accidental human exposure to such agents as primate malaria parasites (12) and a simian immunodeficiency virus (SIV) (16). The potential for exchange through xenotransplantation has been discussed (17), and infection from vaccine contaminated with SV40, a primate papovavirus, led to the exposure of millions of persons in the 1950s (15). Conversely, pathogen transmission from humans to nonhuman primates places both captive and wild animals at serious risk for diseases such as measles and tuberculosis (TB), which are deadly in many nonhuman primate species. In general, as levels of interaction increase, so does pathogen exchange, resulting in further risks to both humans and nonhuman primates.
The need for improved surveillance and basic research on emerging infectious disease is well documented (3,18-20). Wild primates can serve as sentinels by signaling which pathogens pose a risk for humans in the immediate area (21) as well as in distant countries (5). Here, we describe potential benefits of incorporating wild populations into emerging infectious disease research.
Nonhuman primates are infected with the closest relatives of important human pathogens. The construction of molecular phylogenies has played an important role in studying the evolution and classification of many pathogens. While trees that result from these analyses must be interpreted with care, they can provide valuable information on the history of pathogens. Furthermore, the selection of genes that are evolving at the appropriate rate should allow phylogenetic analyses to assess both ancient and more recent epidemic origins (22).
Human herpes simplex virus infection is common in nonhuman primates and may reflect contact with humans. A study of viral infection in nonhuman primates found that chimpanzees and gorillas were seropositive to human herpes simplex virus-1 and -2 strains, but orangutans and gibbons were not (23).
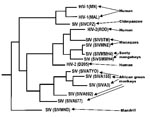
Research on SIV phylogeny (24,25) has shown that HIV-1 and HIV-2 are each more closely related to primate pathogens than they are to one another. HIV-1 is in a group with SIVCPZ (26), a chimpanzee (Pan troglodytes) virus, while HIV-2 falls within a clade consisting of West African primate viruses (Figure 1; 24). In fact, Mindell (27) has argued that HIVs and SIVs should be referred to as `primate immunodeficiency viruses' to more accurately reflect their heritage. Preliminary evidence suggests that the origins of global T-cell lymphotropic virus-1 subtype diversity may be analogous, leading Liu et al. (28) to propose that HTLV-I subtypes emerged from three separate nonhuman primate reservoirs.
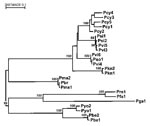
This pattern is not unique to viruses, as demonstrated through decades of research on primate malarias. More than 26 species of Plasmodia infect primates (12,29). Both morphologic and molecular analyses show human and nonhuman primate malarias interdigitating on phylogenetic trees (Figure 2; 30,33). Preliminary evidence suggests that this group of parasites has a range of coevolutionary scenarios, including the speciation of P. vivax and related parasites in Asian primates, the recent exchange of parasites between humans and New World monkeys (30), and perhaps an ancient exchange of a falciparum-like parasite from a bird or lizard to an African hominoid (31,34,35). Further analyses are likely to lead to surprises. For example, recent research has shown that the diversity of human P. vivax (previously considered to be a single species) also includes a P. vivax-like parasite, a widespread pathogen, which is most closely related to P. simiovale, a primate malaria of Asian macaques (Macaca sp.) (30,36).
A major limitation of these studies is that they rely on very small numbers of nonhuman primate isolates only two SIVcpz isolates for the HIV-1 clade, a single P. simiovale isolate for malaria phylogeny. In addition, very few data are available on the distribution of these pathogens in wild populations; more data on the distribution and extent of shared pathogens should provide clues to origins (37), thereby pointing to general conditions that may contribute to disease emergence.
Molecular phylogenies can also play a direct role in the control of pathogens, for example, in charting their antigenic diversity, a task necessary for vaccine design (38). Because nonhuman primate pathogens are often evolutionary outgroups, vaccines that are targeted at antigens shared by human and nonhuman pathogens should provide more universal coverage. Molecular phylogenies can also assist in determining the rate of evolution of vaccine candidate antigens. Because low rates of mutation often indicate selective constraint, this technique may point to candidate antigens that cannot easily mutate to evade vaccine-induced antibodies. Similar analyses identifying highly constrained gene products or metabolic pathways as targets for drug development may contribute to slowing the emergence of drug resistance.
The study of pathogen transmission has been encumbered by the use of inappropriate terminology. Such terms as "primary hosts," "reservoir hosts," "carriers," "arthroponoses," "xenonoses," and "zoonoses" pose premature assumptions and belie aspects of the origins and natural transmission cycles of pathogens. The factors that determine pathogen viability within a vertebrate host may correspond only roughly, or not at all, to species boundaries. Primate pathogens do not adhere to the faithfully maintained sanctity of the distinction between humans and nonhumans.
The misconception of an evolutionary trend toward increasing host specificity (39) has contributed to the belief that pathogen exchange should be rare. An apparently restricted host range, however, may be the product of specific ecologic conditions and not of an intrinsic characteristic of the pathogen. In many cases, ecologic changes can broaden the host range of a pathogen. For example, the filarid worm Loa loa remains outside human populations, primarily because of vector behavior (40); changes in ecology, such as the availability of a novel host, can change vector behavior and expand the pathogen's host range. Similar phenomena may have played a role in the emergence of a range of flaviviruses (e.g., yellow fever, dengue, and Japanese encephalitis) from primarily forest-dwelling nonhuman primate cycles. For these flaviviruses, primate populations may also continue to play a role by introducing novel genetic variants, which, at least in the case of dengue, may be involved in pathogenesis.
Another misconception is that primate populations are too sparse to maintain human pathogens. A number of variables influence whether or not a pathogen is maintained in a given population. The capacity for latency, for example, may decrease the host population size necessary to maintain pathogens. This characteristic has evolved independently in pathogens as diverse as herpesviruses and Plasmodium and may help explain sustained transmission in hosts with low population density. Orangutans (Pongo pygmaeus), for example, are thought to act as hosts to two distinct Plasmodium species (41), despite an estimated population size of two per km2. While molecular phylogenies have not yet been used to verify that these parasites are unique, the presence of a dormant hypnozoite stage might allow for sustained transmission even at this extreme. Where human and nonhuman hosts overlap, both must be factored into epidemiologic models. Small populations of nonimmune humans alone may not be capable of maintaining a pathogen, but when nearby nonhuman hosts are considered, a critical population size may be reached.
Surveys to assess pathogen prevalence among nonhumans can play an important role in control strategies. Eradication programs must consider animal reservoirs; even if complete human coverage is achieved, long-term reemergence from animal reservoirs can undo the best eradication efforts. For example, it is not known if poliovirus infections can be maintained in nonhuman primate populations. Seroprevalence surveys conducted before and after eradication may prove invaluable.
Accidental exposure to infected laboratory workers has led to poliovirus infections of chimpanzees and gorillas since the 1940s (1). Poliovirus can infect not only our closest living relatives, chimpanzees, gorillas (Gorilla gorilla), and orangutans (Pongo pygmaeus) (2), but also more distantly related anthropoids like the colobus monkeys (e.g., Colobus abyssinicus kikuyuensis [=guereza]) (42). Antibodies and shed virus have also been found in recently imported animals (8), and some chimpanzees may act as symptomless carriers (2). Long-term research by Jane Goodall on wild Tanzanian chimpanzees documented the potential for transmission of poliovirus (or a similar virus) in free-ranging chimpanzee populations (43). Since no samples were collected, it is impossible to determine if the epidemic described by Goodall was part of a natural chimpanzee cycle or the result of introduction from local human populations or researchers. As poliovirus eradication efforts intensify, it may be useful to monitor virus prevalence in humans living near primate habitats.
Control efforts that rely on antimicrobial drugs must also take into account the potential for nonhuman primate reservoirs. Despite demonstrations that mass administration of diethylcarbamazine citrate successfully controlled Brugia malayi (a filarid worm), Mak et al. found a high prevalence of B. malayi after a large-scale administration of chemotherapy (44). Research showed that even though periodic prevalence of B. malayi decreased, subperiodic prevalence remained high. The maintenance of subperiodic B. malayi was eventually attributed to mosquitoes infected by leaf monkeys (Presbytis obscura). In this particular free-ranging primate host, approximately 83% of the monkeys were infected (44).
Nonhuman "reservoirs" may also confer potential benefits. It is at least theoretically possible that nonhuman primate populations may provide a barrier to the spread of drug-resistant pathogens; while these pathogens benefit from resistance in environments where drug pressure exists, drug resistance can be costly, and resistant pathogens may not compete effectively against susceptible `wild-type' pathogens in the absence of drug pressure. As drug-free populations, reservoirs may provide havens for susceptible pathogens, thereby decreasing the rate at which drug-resistant genes spread and increasing the rate at which susceptibility may return after drug pressure ends. This hypothesis may help explain why the rates of drug-resistant gram-negative enteric bacteria of wild baboons (Papio cynocephalus) living with limited human contact are significantly lower than those of baboons living with human contact (45).
In tropical lowland forests, which contain the greatest biodiversity of terrestrial habitats (46), exist rarely seen or unknown pathogens with the potential to enter human populations. These pathogens may affect residents of and visitors to forested regions (21) and act as the source of introduction of infectious agents to distant susceptible populations (47). Increasing human contact with forested systems almost certainly leads to a corresponding increase in the emergence of infections in the human population. Nevertheless, predicting which pathogens humans may encounter and be susceptible to remains a methodologic challenge.
Surveillance methods for predicting emerging pathogens include surveillance of vectors or forest-dwelling human populations and wildlife epidemiology (epidemiologic study of infections in wild populations) (48). These approaches have limitations. While vector sampling may prove the easiest method for widespread surveillance, the pathogens identified from vectors may be difficult or impossible to culture. Even when successful, vector sampling is likely to identify a range of pathogens, only some of which may infect humans. Studies of human populations, while providing valuable information, are limited to regions in which forest-dwelling human populations exist.
Epidemiologic research among free-ranging primate populations has the potential to predict which pathogens might enter human populations as contact with forested regions increases. In addition to their physiologic similarities to humans, primates have other characteristics that contribute to their accumulation of infectious agents. Primates live primarily in forested environments (49); in general, they have large bodies and live in large groups characteristics that may attract vectors (50). Furthermore, dependence on fruit, a characteristic of most primates, requires mobility (both terrestrially and arboreally), a trait that may increase exposure to pathogens (51,52).
Despite the lack of organized attempts to document the distribution of pathogens in wild populations, recognized "die-offs" in wild primate populations have played an important role in identifying novel pathogens. In 1956, for example, a novel flavivirus was identified through the investigation of large-scale deaths of bonnet macaques (Macaca radiata) and hanuman langurs (Presbytis entellus) in the Kyanasur Forest of India (Seymour, 1981, cited in [2]) caused by Kyanasur Forest virus. More recently, in 1995, deaths in a chimpanzee population studied by Christoph Boesch in the Täi Forest, Côte d'Ivoire, and a single human case following a necropsy led to the identification of a novel strain of Ebola virus (9,53). The single human case in the Swiss researcher foreshadowed the localized mini-outbreak of Ebola hemorrhagic fever in Mayibout, a village in the northeast of Gabon in January 1996. The Gabon epidemic was linked to the handling, preparation, and consumption of a chimpanzee that had been found dead; 29 of 37 identified cases involved exposure to the dead chimpanzee (54). Close monitoring of such populations, as is being conducted in the Täi Forest, has the potential to identify emergence-linked behavior, such as the consumption of specific plants or insects, which may lead to the still elusive reservoir of Ebola virus. Considering the exceptionally small percentage of wild primate populations under long-term study, these examples represent only the tip of the iceberg. More systematic monitoring of wild primate populations will likely provide a substantial payoff in our understanding, identification, and possible control of novel pathogens, both for humans and endangered primates.
Surveillance for certain types of human-nonhuman primate contact may be particularly useful. Hunting, which involves tracking, capturing, handling, transporting, preparing, and consuming meat, may play a particularly important role in pathogen exchange. In addition to the recent evidence of hunting-mediated Ebola transmission, the hunting of a red colobus (Colobus pennanti oustaleti [=badius]) has been implicated in a localized epidemic of monkeypox, an orthopoxvirus similar to smallpox, which continued for four generations of human-to-human contact (55). Another example is the increased risk for feline plague among cats that hunt rodents (7). Necropsies share many characteristics with hunting and are appropriately considered a high-risk activity.
Bites from wild primates may also play a role in the transmission of certain pathogens. For example, chimpanzee-to-human transmission of monkeypox occurred when a wild chimpanzee bit a 2-year-old girl (6). Further sampling demonstrated high prevalence in forest squirrel populations (up to 49% among Funisciurus lemniscatus) (56), underlining the need for comprehensive studies before determining the ultimate source and reservoir of pathogens. Bites and scratches have transmitted pathogens to laboratory workers. The transmission of B-virus, a herpesvirus infecting rhesus macaques (Macaca mulatta), has caused death in 18 of 24 known human cases (57). Surveillance and education in human populations that hunt nonhuman primates, as well as follow-up of reported primate bites in nonlaboratory settings, may be indicated.
The potential for disease emergence and reemergence depends on the interaction of complex social, ecologic, and genetic factors at the host, vector, and pathogen levels (58). Because wild populations of primates display diverse social behavior and live in a range of ecologic environments, they exemplify natural transmission. Furthermore, monitoring pathogens in wild primate populations does not involve the treatment or behavior change interventions that monitoring pathogens in humans requires. Exceptions include the increasingly common need for wildlife medicine to maintain the health of endangered species and decrease the impact of pathogens from humans and domestic animals on free-ranging animals (59). Nevertheless, for many pathogens, basic epidemiologic research, and not treatment, remains the primary goal of wildlife medicine (59).
Studies of wild populations can highlight factors associated with the pathogen exchange across species boundaries. Some parasites, for example, the chewing lice–pocket gopher system, complete their entire life cycle on a single host. This high level of host specificity may contribute to the close cospeciation between pocket gophers and their respective lice; the phylogenetic trees of host and parasite are nearly mirror images (60). Nevertheless, such host-specific parasites may be the exception rather than the rule. Many intestinal parasites, for example, seem to be generalists. One epidemiologic study of wild primates in the Kibale Forest National Park, Uganda, evaluated the role of primate distribution on the distribution of intestinal amoebas (61); the park contains a number of spatially separate primate groups, each of which consists of multiple primate species. Most variation in amoeba prevalence was explained by group membership, and little was explained by species, which suggests that these parasites treated the primate groups as biologic islands (62), spreading easily among diverse members of the same island but rarely spreading to new islands.
Long-term behavioral research combined with occasional pathogen sampling may provide valuable data. Long-term research on the SIVs of East African primates, for example, has provided evidence for a recent cross-species transmission of SIV between baboons and African grivet monkeys (Cercopithecus aethiops aethiops) (62). This research has documented the incidence of SIVagm among Ethiopian grivet monkeys for more than 20 years (63). By examining prevalence in age groups over time, this research demonstrated the minimal impact of SIVagm on the survival of grivets, the predominance of sexual transmission, and the lack of maternal transmission.
An ecologic approach to pathogen transmission can benefit our understanding of emerging diseases. By determining how humans become a part of the life cycle of pathogens rather than how pathogens enter human populations, we can better understand the factors associated with emergence and improve the quality of public health responses. How humans become part of the life cycle of pathogens depends on human migrations, environmental changes, and cultural and social factors, in the context of evolutionary history of the pathogens, vectors, and hosts, which make up an infectious system. Ecologic and evolutionary studies of wild animals in general, and primate populations in particular, can address questions arising from these complex interactions.
Traditionally, the study of wild populations of primates has been the domain of primatologists and wildlife veterinarians, who have worked to overcome logistic difficulties in the field, develop methods, and address ethical issues. Opportunities exist for collaborative work on infectious disease; the benefits of such efforts are considerable. The translocation of animals from vulnerable forest fragments to forest reserves, an increasingly common conservation effort, is an opportunity for pathogen sampling. Other possibilities are provided by collaboration with long-term behavioral research on free-ranging animals. While behavioral research sites may only provide fecal and urine samples, the study of intestinal parasites is often possible; recent advances in urinalysis demonstrate that urine may be a source of both antigen and antibody from systemic infections, such as malaria (64). The distribution of appropriate necropsy protocols and sample collection kits would improve data collection and decrease risks associated with necropsies. Urgently needed are strategies for noninvasive remote diagnosis. For example, combined with sensitive molecular diagnostics, a remote tissue-biopsy dart system (65) may have potential for obtaining epidemiologic samples.
Efforts to preserve endangered primates and monitor disease emergence have some common objectives. Pathogen exchange is a two-way street, and exposure to human pathogens poses a serious threat to endangered animals (11). While the main focus of this article has been risk to humans, nonhuman primates are frequently more threatened by microorganisms indigenous to humans than vice versa (2). TB, which is often fatal to nonhuman primates, represents a serious threat to laboratory primate communities, which commonly are infected by humans and can occasionally reinfect laboratory workers (66). TB is prevalent in wild populations, as demonstrated by the presence in wild olive baboons (Papio cynocephalus) of Mycobacterium bovis infection, an infection that most likely originated from cattle (67).
Both hunting and forest encroachment threaten endangered primates and increase the possibility of human and nonhuman infectious disease emergence. Research on the pathogens of primates and humans on forest boundaries helps assess risks to wild primates and to humans. In addition to their value in the study of infectious disease and human evolution, many primates are valuable natural resources in their home countries. The veterinary expertise and wildlife management skills of conservation organizations can both supplement the basic pathogen research and control work of the public health community and benefit from it.
The examination of pathogen exchange in regions of host overlap may identify social factors that influence pathogen emergence. Data on forest use by human communities surrounding forest reserves and levels of crop-raiding by nonhuman primates have been collected as part of ongoing conservation projects. A comparison of these data with human and nonhuman primate pathogen prevalence may provide a measure of forest-oriented behavioral risks. Further research comparing infection rates among hunters and nonhunters could confirm the findings and determine the role of behavioral control measures to decrease risk.
Much remains to be done. Recent evidence has suggested that some wild primates self-medicate with plants in their environment (68). An understanding of the underlying wildlife epidemiology, therefore, combined with long-term dietary data and analysis of plant chemistry, may lead to new chemotherapeutic drugs (69). In addition, research on the dynamics of primate pathogens in their natural hosts may elucidate novel host resistance mechanisms. In particular, evidence of impaired host survival and reproduction, as well as long-term host-parasite association, may point to hosts that are likely to have evolved genetically mediated resistance. Such resistance mechanisms in humans (70) have begun to play an increasingly important role in vaccine and drug development (71,72). While the findings from captive primate studies have played an important role in medicine in the 20th century, this period has also been marked by a notable absence of research on the basic ecology of disease systems. Perhaps by learning from primates in their natural environments we may better prepare ourselves for the disease threats to humans and wildlife populations in the coming century.
Nathan Wolfe is a doctoral candidate in the Department of Immunology and Infectious Disease at the Harvard School of Public Health. Mr. Wolfe has conducted research on self-medicating behavior among chimpanzees and mountain gorillas in Uganda and is collaborating with Drs. Altaf Lal and Ananias Escalante on malaria evolution research.
Acknowledgment
We thank Adria Tassy Prosser and Daniel G. Colley for valuable suggestions and Mary Bartlett for editorial assistance. Nathan Wolfe is supported by a Taplin Graduate Fellowship and a National Science Foundation-Predoctoral Fellowship. Ananias A. Escalante is supported by the ASM/NCID Postdoctoral Research Associates Program.
References
- Ruch TC. Diseases of laboratory primates. Philadelphia: W.B. Saunders Company; 1959.
- Brack M. Agents transmissible from simians to man. Berlin: Springer-Verlag; 1987.
- Morse SS. Emerging viruses. In: Morse SS, editor. Emerging viruses. New York: Oxford University Press, Inc.; 1993.
- Levins R, Awerbuch T, Brinkmann U, Eckardt I, Epstein P, Makhoul N, The emergence of new diseases. Am Sci. 1994;82:52–60.
- Wilson ME, Levins R, Spielman A. Disease in evolution: global changes and emergence of infectious diseases. Ann N Y Acad Sci. 1994;:740.
- Mutombo M, Arita I, Jezek Z. Human monkeypox transmitted by a chimpanzee in a tropical rain-forest area of Zaire. Lancet. 1983;34:735–7. DOIGoogle Scholar
- Eidson M, Tierney LA, Rollag OJ, Becker T, Brown T, Hull HF. Feline plague in New Mexico: risk factors and transmission to humans. Am J Public Health. 1988;78:1333–5. DOIPubMedGoogle Scholar
- Douglas JD, Soike KF, Raynor J. The incidence of poliovirus in chimpanzees (Pan troglodytes). Lab Anim Care. 1970;20:265–8.PubMedGoogle Scholar
- Morell V. Chimpanzee outbreak heats up search for Ebola origin. Science. 1995;268:974–5. DOIPubMedGoogle Scholar
- Meyers WM, Gormus BJ, Walsh GP, Baskin B, Hubbard GB. Naturally acquired and experimental leprosy in nonhuman primates. Am J Trop Med Hyg. 1991;44:24–7.PubMedGoogle Scholar
- McCallum H, Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol Evol. 1995;10:190–4. DOIGoogle Scholar
- Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. Bethesda: U.S. Department of Health, Education, and Welfare; 1971.
- Beverley-Burton M, Crichton VF. Attempted experimental cross infections with mammalian guinea-worms, Drancunculus spp. (Nematoda: Dracunculoidea). Am J Trop Med Hyg. 1976;25:704–8.PubMedGoogle Scholar
- Fuller GK, Lemma A, Haile T. Schistosomiasis in Omo National Park of Southwest Ethiopia. Am J Trop Med Hyg. 1979;28:526–30.PubMedGoogle Scholar
- Shah KV. A review of the circumstances and consequences of simian virus SV40 contamination of human vaccines. Symposium on Continuous Cell Lines as Substrates for Biologicals. Developments in biological standardization, Vol. 70; 1989.
- Khabbaz RF, Heneine W, George JR, Parekh B, Rowe T, Woods T, Brief report: infection of a laboratory worker with simian immunodeficiency virus. N Engl J Med. 1994;330:172–7. DOIPubMedGoogle Scholar
- Michler RE. Xenotransplantation: risks, clinical potential, and future prospects. Emerg Infect Dis. 1996;2:64–70. DOIPubMedGoogle Scholar
- Berkelman RL, Pinner RW, Hughes JM. Addressing emerging microbial threats in the United States. JAMA. 1996;275:315–7. DOIPubMedGoogle Scholar
- Lederberg J, Shope RE, Oaks SC. Emerging infections: microbial threats to health in the United States. Washington: National Academy Press; 1992.
- LeDuc JW. World Health Organization strategy for emerging infectious diseases. JAMA. 1996;275:318–20. DOIPubMedGoogle Scholar
- Meslin FX. Surveillance and control of emerging zoonoses. World Health Stat Q. 1992;45:200–7.PubMedGoogle Scholar
- Holmes ED, Nee S, Rambaut A, Garnett GP, Harvey PH. Revealing the history of infectious disease epidemics through phylogenetic trees. Philos Trans R Soc Lond B Biol Sci. 1995;349:33–40. DOIPubMedGoogle Scholar
- Kalter SS, Heberling RL, Cooke AW, Barry JD, Tian PY, Northam WJ. Viral infections of non-human primates. Lab Anim Sci. 1997;47:461–7.PubMedGoogle Scholar
- Myers G, MacInnes K, Korber B. The emergence of simian/human immunodeficiency viruses. AIDS Res Hum Retroviruses. 1992;8:373–86. DOIPubMedGoogle Scholar
- Peeters M, Fransen K, Delaporte E, Van Den Haesevelde M, Gershy-Damet GM, Kestens L, Isolation and characterization of a new chimpanzee lentivirus (simian immunodeficiency virus isolate cpz-ant) from a wild-captured chimpanzee. AIDS. 1992;6:447–51.PubMedGoogle Scholar
- Huet TR, Cheynier A, Meyerhans A, Roelants G, Wain-Hobson S. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature. 1990;345:356–459. DOIPubMedGoogle Scholar
- Mindell DP. Positive selection and rates of evolution in immunodeficiency viruses from humans and chimpanzees. Proc Natl Acad Sci U S A. 1996;93:3284–8. DOIPubMedGoogle Scholar
- Liu HF, Goubau P, Van Brussel M, Van Laethem K, Chen YC, Desmyter J, The three human T-lymphotropic virus type I subtypes arose from three geographically distinct simian reservoirs. J Gen Virol. 1996;77:359–68. DOIPubMedGoogle Scholar
- Collins WE. Major animal models in malaria research: simian. In: Wernsdorfer WH, editor. Malaria: principles and practice of malariology. Edinburgh: Churchill Livingstone; 1988.
- Escalante AA, Barrio E, Ayala FJ. Evolutionary origin of human and primate malarias: evidence from the circumsporozoite protein gene. Mol Biol Evol. 1995;12:616–26.PubMedGoogle Scholar
- McCutchan TF, Kissinger JC, Touray MG, Rogers MJ, Li J, Sullivan M, Comparison of circumsporozoite proteins from avian and mammalian malaria: biological and phylogenetic implications. Proc Natl Acad Sci U S A. 1996;93:11889–94. DOIPubMedGoogle Scholar
- Tajima F, Nei M. Estimation of evolutionary distance between nucleotide sequences. Mol Biol Evol. 1984;1:269–85.PubMedGoogle Scholar
- Qari SH, Shi YP, Pieniazek NJ, Collins WE, Lal AA. Phylogenetic relationship among the malaria parasites based on small subunit rRNA gene sequences: monophyletic nature of the human malaria parasite, Plasmodium falciparum. Mol Phylogenet Evol. 1996;6:157–65. DOIPubMedGoogle Scholar
- Waters AP, Higgins DG, McCutchan TF. Evolutionary relatedness of some primate models of Plasmodium. Mol Biol Evol. 1993;10:914–23.PubMedGoogle Scholar
- Escalante AA, Ayala FJ. Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences. Proc Natl Acad Sci U S A. 1994;91:11373–7. DOIPubMedGoogle Scholar
- Qari SH, Shi YP, Povoa MM, Alpers MP, Deloron P, Murphy GS, Global occurrence of Plasmodium vivax-like human malaria parasite. J Infect Dis. 1993;168:1485–9.PubMedGoogle Scholar
- Desrosiers RC. HIV-1 origins: a finger on the missing link. Nature. 1990;345:288–9. DOIPubMedGoogle Scholar
- Peeters M, Janssens W, Fransen K, Brandful J, Heyndrickx L, Koffi K, Isolation of simian immunodeficiency viruses from two sooty mangabeys in Côte d'Ivoire: virological and genetic characterization and relationship to other HIV type 2 and SIVsm/mac strains. AIDS Res Hum Retroviruses. 1994;10:1289–94. DOIPubMedGoogle Scholar
- Brooks DR, McLennan DA. Parascript: parasites and the language of evolution. Washington: Smithsonian Institution Press; 1993.
- Gouteux JP, Noireau F. The host preferences of Chrysops silacea and C. dimidiata. Diptera: Tabanidae in an endemic area of Loa loa in the Congo. Ann Trop Med Parasitol. 1989;83:167–72.PubMedGoogle Scholar
- Peters W, Garnham PCC, Rajapaksa N, Cheong WH, Cadigan FC. Malaria of the Orangutan in Borneo. Philos Trans R Soc Lond B Biol Sci. 1976;275:439–82. DOIPubMedGoogle Scholar
- Suleman MA, Johnson BJ, Tarara R, Sayer PD, Ochieng DM, Muli JM, An outbreak of poliomyelitis caused by poliovirus type I in captive black and white colobus monkeys (Colobus abyssinicus kikuyuensis) in Kenya. Trans R Soc Trop Med Hyg. 1984;78:665–9. DOIPubMedGoogle Scholar
- Goodall J. Population dynamics during a 15 year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Z Tierpsychol. 1983;61:1–60.
- Mak JW, Cheong WH, Yen PK, Lim PK, Chan WC. Studies on the epidemiology of subperiodic Brugia malayi in Malaysia: problems in its control. Acta Trop. 1982;39:237–45.PubMedGoogle Scholar
- Rolland RM, Hausfater G, Marshall B, Levy SB. Antibiotic-resistant bacteria in wild primates: increased prevalence in baboons feeding on human refuse. Appl Environ Microbiol. 1985;49:791–4.PubMedGoogle Scholar
- Turner IM. Species loss in fragments of tropical rain forest: a review of the evidence. J Appl Ecol. 1996;33:200–9. DOIGoogle Scholar
- Wilson ME. Travel and the emergence of infectious diseases. Emerg Infect Dis. 1995;1:39–46. DOIPubMedGoogle Scholar
- Grenfell BT, Dobson AP, eds. Ecology of infectious diseases in natural populations. Cambridge: Cambridge University Press; 1995.
- Fleagle JG, Reed KE. Comparing primate communities: a multivariate approach. J Hum Evol. 1996;30:489–510. DOIGoogle Scholar
- Davies CR, Ayres JM, Dye C, Deane LM. Malaria infection rate of Amazonian primates increases with body weight and group size. Funct Ecol. 1991;5:655–62. DOIGoogle Scholar
- Freeland WJ. Pathogens and the evolution of primate sociality. Biotropica. 1976;8:12–24. DOIGoogle Scholar
- Loehle C. Social barriers to pathogen transmission in wild animal populations. Ecology. 1995;76:326–35. DOIGoogle Scholar
- Le Guenno B, Formentry P, Wyers M, Gounon P, Walker F, Boesch C. Isolation and partial characterization of a new strain of Ebola virus. Lancet. 1995;345:1271–444. DOIPubMedGoogle Scholar
- World Health Organization. Outbreak of Ebola haemorrhagic fever in Gabon officially declared over. Wkly Epidemiol Rec. 1996;71:125–6.
- Jezek Z, Arita I, Mutombo M, Dunn C, Nakano JH, Szczeniowski M. Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol. 1986;123:1004–12.PubMedGoogle Scholar
- Fenner SS. Human monkeypox, a newly discovered human virus disease. In: Morse SS, editor. Emerging viruses. New York: Oxford University Press, Inc.; 1993. p. 176-183.
- Palmer AE. Herpesvirus simiae: historical perspective. J Med Primatol. 1987;16:99–130.PubMedGoogle Scholar
- Schrag SJ, Wiener P. Emerging infectious disease: what are the relative roles of ecology and evolution? Trends Ecol Evol. 1995;10:319–24. DOIGoogle Scholar
- Karesh WB, Cook RA. Applications of veterinary medicine to in situ conservation efforts. Oryx. 1995;29:244–52. DOIGoogle Scholar
- Hafner MS, Page RDM. Molecular phylogenies and host-parasite cospeciation: gophers and lice as a model system. Philos Trans R Soc Lond B Biol Sci. 1995;349:77–83. DOIPubMedGoogle Scholar
- Freeland WJ. Primate social groups as biological islands. Ecology. 1979;60:719–28. DOIGoogle Scholar
- Jin MJ, Rogers J, Phillips-Conroy JE, Allan JS, Desrosiers RC, Shaw GM, Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J Virol. 1994;68:8454–60.PubMedGoogle Scholar
- Jolly CJ, Phillips-Conroy JE, Turner TR, Broussard S, Allan JS. SIV-agm incidence over two decades in a natural population of Ethiopian grivet monkeys (Cercopithecus aethiops aethiops). J Med Primatol. 1996;25:78–83.PubMedGoogle Scholar
- Rodriguez del Valle M, Quakyi IA, Amuesi J, Quaye JT, Nkrumah FK, Taylor DW. Detection of antigens and antibodies in the urine of humans with Plasmodium falciparum malaria. J Clin Microbiol. 1991;29:1236–42.PubMedGoogle Scholar
- Karesh WB, Smith F, Frazier-Taylor H. A remote method for obtaining skin biopsy samples. Conserv Biol. 1987;1:261–2. DOIGoogle Scholar
- Centers for Disease Control and Prevention. Tuberculosis in imported nonhuman primatesUnited States, June 1990-May 1993. MMWR Morb Mortal Wkly Rep. 1993;42:572–6.PubMedGoogle Scholar
- Tarara R, Suleman MA, Sapolsky R, Wabomba MJ, Else JG. Tuberculosis in wild olive baboons, Papio cynocephalus anubis (Lesson), in Kenya. J Wildl Dis. 1985;21:137–40.PubMedGoogle Scholar
- Clayton DH, Wolfe ND. The adaptive significance of self-medication. Trends Ecol Evol. 1995;8:60–3. DOIGoogle Scholar
- Robles M, Aregullin M, West J, Rodriguez E. Recent studies on the zoopharmacognosy, pharmacology and neurotoxicology of sesquiterpene lactones. Planta Med. 1995;61:199–203. DOIPubMedGoogle Scholar
- Hill AV, Yates SN, Allsopp CE, Gupta S, Gilbert SC, Lalvani A, Human leukocyte antigens and natural selection by malaria. Philos Trans R Soc Lond B Biol Sci. 1994;346:379–85. DOIPubMedGoogle Scholar
- Skolnick AA. Newfound genetic defect hints at clues for developing novel antimalarial agents. JAMA. 1993;269:1765. DOIPubMedGoogle Scholar
- Stephenson J. Findings on host resistance genes for infectious diseases are pointing the way to drugs, vaccines. JAMA. 1996;275:1464–5. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 4, Number 2—June 1998
| EID Search Options |
|---|
|
|
|
|
|
|