Volume 5, Number 3—June 1999
Dispatch
Fatal Case Due to Methicillin-Resistant Staphylococcus aureus Small Colony Variants in an AIDS Patient
Cite This Article
Citation for Media
Abstract
We describe the first known case of a fatal infection with small colony variants of methicillin-resistant Staphylococcus aureus in a patient with AIDS. Recovered from three blood cultures as well as from a deep hip abscess, these variants may have resulted from long-term antimicrobial therapy with trimethoprim/sulfamethoxazole for prophylaxis of Pneumocystis carinii pneumonia.
Staphylococcus aureus causes acute and often fatal infections. Small colony variants (SCVs), which are subpopulations of S. aureus, are implicated in persistent and recurrent infections (in particular osteomyelitis, septic arthritis, respiratory tract infections in patients with cystic fibrosis, and deep-seated abscesses) (1-4). These phenotypic variants produce small, slow-growing, nonpigmented, nonhemolytic colonies on routine culture media, making correct identification difficult for clinical laboratories. Biochemical characterization of these variants suggests that they are deficient in electron transport activity (5).
We report a fatal case of a persistent deep-seated hip abscess due to methicillin-resistant S. aureus SCVs that led to osteomyelitis and bloodstream infection in a patient with AIDS.
A 36-year-old man with AIDS came to the Cologne University Hospital, Cologne, Germany, in June 1997 with fever and progressive pain (of 6 weeks duration) in his right hip. HIV infection had been diagnosed in 1986. In 1994, his CD4 cell count was 250/µL, and oral zidovudine therapy was started. His medical history included Pneumocystis carinii pneumonia, pulmonary tuberculosis, and recurrent oral thrush; his medication included zidovudine, lamivudine, fluconazole, and trimethoprim/sulfamethoxazole. In September 1996, he was in a traffic accident and had severe cerebral trauma resulting in spastic hemiparesis with occasional seizures. After an intramuscular injection 2 months before admission, pus was surgically drained to treat recurrent abscesses of his right hip. Specimens for culture were not obtained.
Physical examination found limited mobility of his right thigh and a tender, nondraining scar at the site of surgical drainage. Neither warmth nor swelling was observed over his right hip. Vital signs were temperature, 38.2°C; respiration rate, 28; and heart rate, 108. He was awake and alert and had spastic paresis in his right arm.
Laboratory studies performed on admission showed hemoglobin, 10.8 g/dL; leukocyte count, 3,000/µL with a normal differential; CD4 cell count, 20/µL; platelet count, 131,000/µL; C-reactive protein, 184 mg/L; and alkaline phosphatase, 1490 U/L. Radiographs of the chest and a plain film of the pelvis were normal. A triple-phase bone scan showed an area of minor tracer accumulation in the acetabulum region of the right hip. Blood cultures were drawn, but antimicrobial therapy was withheld until culture results became available.
On hospital day 2, one of two blood cultures drawn on admission yielded nonhemolytic staphylococci that were clumping factornegative. The organisms were initially misidentified as coagulase-negative staphylococci and were considered contaminants. Empiric antistaphylococcal therapy with clindamycin (600 mg q8hr) was instituted. On hospital day 4, two sets of blood cultures obtained on hospital day 2 yielded phenotypically identical organisms, which on the basis of a positive tube coagulase test were identified as oxacillin-resistant S. aureus. The colony morphology was suggestive of an SCV of S. aureus. The patient was started on parenteral vancomycin treatment (1 g q12hr). However, his condition deteriorated rapidly, and he died of refractory septic shock 6 days after admission.
Autopsy showed a large (12 x 10 x 8 cm), deep-seated abscess of the right hip and osteomyelitis of the ischial tuberosity. Both SCVs and typical large colony forms of S. aureus were cultured from postmortem specimens of the abscess and the bone.
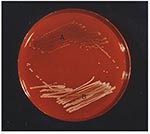
S. aureus SCVs were recovered from one of two blood culture sets obtained on admission and from two of four blood culture sets obtained on hospital day 2. Growth was not detected until the blood culture bottles had been incubated 24 hours. S. aureus with a normal phenotype was recovered from nose and throat specimens but not from blood cultures, whereas both SCVs and typical S. aureus phenotypes were isolated from the deep hip abscess (Figure 1) before death, as well as from a postmortem specimen. All isolates were clumping factor–negative but showed a delayed positive reaction in the tube-coagulase test at 24 hours. The results of the ID 32 staph test did not unambiguously identify SCVs as S. aureus because the tests for urease and trehalose were negative. Both the nuc gene and the coa gene were identified by polymerase chain reaction (PCR) amplification. Methicillin resistance was confirmed for both small and large colony forms by PCR amplification of the mecA gene.
When cultured without supplementation, all SCVs were nonpigmented and nonhemolytic. Supplementation with hemin, thymidine, or menadione identified two SCVs showing thymidine auxotrophy and a combined thymidine and menadione auxotrophy, respectively. All SCVs were stable on repeated subculturing.
Epidemiologic typing by PCR analysis of inter-IS256 spacer length polymorphisms (Figure 2) and pulsed-field gel electrophoresis of genomic DNA (data not shown) showed identical banding patterns for both SCVs and large colony forms, which indicates that the phenotypically different S. aureus isolates represented a single strain. Antimicrobial susceptibility testing was performed by microbroth dilution, according to the National Committee for Clinical Laboratory Standards guidelines. Susceptibility to trimethoprim/sulfamethoxazole was tested with Etest (AB Biodisk, Solna, Sweden). In contrast to current standards, the MICs for SCVs were determined after 48 hours of incubation at 35°C. Susceptibility testing showed that all S. aureus isolates were resistant to penicillin (MIC, >8 µg/mL), ampicillin (MIC, >32 µg/mL), oxacillin (MIC, >8 µg/mL), erythromycin (MIC, >32 µg/mL), clindamycin (MIC, >32 µg/mL), ciprofloxacin (MIC, >8 µg/mL), gentamicin (MIC, >500 µg/mL), and trimethoprim/sulfamethoxazole (MIC, >32 µg/mL) and susceptible to vancomycin (MICs, 1-2 µg/mL), teicoplanin (MICs, 0.5-1 µg/mL), and quinupristin/dalfopristin (MICs, 0.5-1 µg/mL). No differences in MICs were observed between S. aureus SCVs and S. aureus isolates with normal phenotype.
To our knowledge, this case represents the first of a serious S. aureus infection in an AIDS patient in which all blood cultures yielded SCVs. The SCVs' unusual morphologic appearance and slow growth delayed the correct identification of these organisms as S. aureus. The empiric antimicrobial regimen in our patient did not include a glycopeptide, because of the low rate of methicillin resistance in community-acquired S. aureus infection in Germany. Appropriate antistaphylococcal therapy was, therefore, not started until hospital day 4. Delayed antimicrobial therapy on day 4 rather than on day 2 may have contributed to the patient's death.
Proctor and colleagues recently reported five cases in which SCVs of S. aureus were implicated in persistent and relapsing infections. They identified only a single case reported in the previous 17 years and ascribed this to insufficient ability of laboratories to identify these organisms (8). In most cases, patients had received antibiotics. Aminoglycoside treatment may have selected for S. aureus SCVs (10), and in cases of osteomyelitis or deep-seated abscesses, persistence of these variants in the intracellular milieu may have permitted evasion of host defenses and allowed for the development of resistance to antimicrobial therapy (7,11). Von Eiff and colleagues recently reported four cases of chronic osteomyelitis due to SCVs of S. aureus in patients who had received gentamicin beads as an adjunct to surgical therapy for osteomyelitis (2). Kahl et al. described persistent infection with S. aureus SCVs in patients with cystic fibrosis (4). All these patients had received long-term trimethoprim/sulfamethoxazole prophylaxis. It may be tempting to speculate that administration of trimethoprim/sulfamethoxazole for prophylaxis against P. carinii pneumonia may have selected for SCVs within the patient's large hip abscess. Further prospective studies are needed to assess the role of S. aureus SCVs in HIV-infected patients on long-term antimicrobial therapy.
Dr. Seifert is assistant professor at the Institute of Medical Microbiology and Hygiene, University of Cologne, Germany. His research interests include the molecular epidemiology of nosocomial pathogens, in particular Acinetobacter species, catheter-related infections, and antimicrobial resistance.
References
- Proctor RA, Balwit JM, Vesga O. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect Agents Dis. 1994;3:302–12.PubMedGoogle Scholar
- von Eiff C, Bettin D, Proctor RA, Rolauffs B, Lindner N, Winkelmann W, Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis. 1997;25:1250–1. DOIPubMedGoogle Scholar
- Spearman P, Lakey D, Jotte S, Chernowitz A, Claycomb S, Stratton C. Sternoclavicular joint septic arthritis with small colony variant Staphylococcus aureus. Diagn Microbiol Infect Dis. 1996;26:13–5. DOIPubMedGoogle Scholar
- Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis. 1998;177:1023–9.PubMedGoogle Scholar
- von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Götz F. A site-directed Staphylococcus aureus hemB mutant is a small colony variant which persists intracellularly. J Bacteriol. 1997;179:4706–12.PubMedGoogle Scholar
- Kloos WE, Bannerman TL. Staphylococcus and micrococcus. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. 6th ed. Washington: American Society for Microbiology; 1995. p. 282-98.
- Balwit JM, van Langevelde P, Vann JM, Proctor RA. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170:1033–7.PubMedGoogle Scholar
- Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. Persistent and relapsing infections associated with small colony variants of Staphylococcus aureus. Clin Infect Dis. 1995;20:95–102.PubMedGoogle Scholar
- Deplano A, Vaneechoutte M, Verschraegen G, Struelens MJ. Typing of Staphylococcus aureus and Staphylococcus epidermidis by PCR analysis of inter-IS256 spacer length polymorphisms. J Clin Microbiol. 1997;35:2580–7.PubMedGoogle Scholar
- Pelletier LL Jr, Richardson M, Feist M. Virulent gentamicin-induced small colony variants of Staphylococcus aureus. J Lab Clin Med. 1979;94:324–34.PubMedGoogle Scholar
- Proctor RA, Kahl B, von Eiff C, Vaudaux PE, Lew DP, Peters G. Staphylococcal small colony variants have novel mechanisms for antibiotic resistance. Clin Infect Dis. 1998;27(Suppl 1):S68–74. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 5, Number 3—June 1999
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Harald Seifert, Institute of Medical Microbiology and Hygiene, University of Cologne, Goldenfelsstraße 19-21, 50935 Cologne, Germany; fax: 49-221-478-3081
Top