Volume 6, Number 6—December 2000
Perspective
Evidence Against Rapid Emergence of Praziquantel Resistance in Schistosoma haematobium, Kenya
Abstract
We examined the long-term efficacy of praziquantel against Schistosoma haematobium, the causative agent of urinary schistosomiasis, during a school-based treatment program in the Msambweni area of Coast Province, Kenya, where the disease is highly endemic. Our results, derived from treating 4,031 of 7,641 children from 1984 to 1993, indicate substantial year-to-year variation in drug efficacy. However, the pattern of this variation was not consistent with primary or progressive emergence of praziquantel resistance. Mathematical modeling indicated that, at current treatment rates, praziquantel resistance will likely take 10 or more years to emerge.
Schistosomiasis remains a public health problem in many regions, including Africa, the Middle East, Asia, and South America (1). For many of the Schistosoma species that infect humans, isoquinolin-4-one, praziquantel, is the only effective drug (2,3). Its minimal side effects and high degree of efficacy against both trematodes and cestodes have made it the drug of choice for many human and veterinary parasitic infections (2,4). However, praziquantel has been in use for more than 20 years (5), and concern is increasing that resistance has emerged, or will soon emerge, in human parasites (6,7).
Loss of praziquantel efficacy would set back helminth control efforts. Many community-based programs depend on praziquantel for treating patients with schistosomiasis, cysticercosis, echinococcosis, and tapeworm and other fluke infections (5,8-13). Concern over possible loss of efficacy prompted the European Commission to establish an International Initiative on Praziquantel Use, which met in February 1998 (14) and again in February 1999 (15). The group reviewed reports of low efficacy in clinical trials in Senegal and Egypt (16-19) and of laboratory isolation of schistosome strains resistant to standard and high doses of the drug (20-23). Although there was no definitive laboratory evidence of genetically transmissible and drug-selectable resistance (as had been demonstrated for the antischistosome drug hycanthone [24]), concern was raised over possible low-level resistance. The need was expressed for continued monitoring for resistant strains under the pressure of widespread praziquantel use (14).
We examined drug efficacy in the community and among schoolchildren given repeated praziquantel treatment for S. haematobium in Coast Province, Kenya. Year-to-year variation in treatment response was assessed, and the likely time-to-emergence of resistance was evaluated through the use of mathematical modeling of resistance-gene transmission in this obligately diecious parasite.
Study Design
The overall goal of the community study was to treat urinary schistosomiasis in schoolchildren (initial N = 3,196) in a nine-village area in Kwale District, Coast Province, Kenya. After oral informed consent was obtained under a human investigations protocol approved by the institutional review boards of University Hospitals of Cleveland and the Ministry of Health, Kenya, case-finding was performed by school-based and follow-up village surveys. Details of the protocols have been published (25-27). In 1984, the initial treatment year, S. haematobium-infected children were randomly assigned to groups for treatment with either praziquantel (Biltricide, Bayer, Leverkusen, Germany), 40 mg/kg once a year, or metrifonate (Bilarcil, Bayer), 10 mg/kg three times a year. In years 2 and 3, the initial treatment was repeated, independent of parasitologic findings. New entrants to the study were assigned randomly to either the praziquantel or metrifonate treatment groups, according to the original 1984 protocol. In 1987, half the 1984 cohort, treated either with praziquantel or with metrifonate, was randomized to receive a single dose of metrifonate, 10 mg/kg, as a "consolidation" treatment. After a 2-year hiatus, annual treatment was resumed in 1989 to 1991; during this period, only children in whose urine samples eggs were identified (egg-positive children) were treated with praziquantel alone.
Infection status was determined by Nuclepore (Whatman, Kent, UK) filtration of two 10-mL samples from stirred midday urine specimens. Infection-associated disease was determined by physical examination, dipstick urine examination for hematuria and proteinuria, and ultrasound examination of the kidneys and bladder (25). Clinical and parasitologic testing was performed each year. Results were coded and entered for analysis in databases at Case Western Reserve University and the Kenyan Ministry of Health.
Data Analysis
Results of annual treatments were scored as "cure" for patients whose status changed from egg-positive to egg-negative, "noncure" for those whose status remained egg-positive, and "infected or reinfected" for those whose status changed from egg-negative to egg-positive between yearly examinations. Because of the skewed distribution of egg counts in the infected population, the effects of treatment on average intensity of infection were assessed by determining the change in geometric mean egg count between examinations. Differences between outcome rates were assessed by the chi-square test with Yates' correction or Fisher's exact test.
Mathematical Modeling
The potential for development of praziquantel resistance in the study population was first estimated by the Hardy-Weinberg equilibrium analysis (28). We then used a deterministic, simultaneous differential equation model of helminth resistance (Appendix , 29). This more advanced model takes into account the skewed (negative binomial) distribution of number of worms in human populations, the obligate sexual reproduction of the parasites, and a possible decrease in fecundity as a result of parasite crowding in heavily infected humans. The model provides estimates of average level of infection in the study population, as well as the prevalence and density of resistant worms over time. Results are shown as three-dimensional graphs of mean numbers of worms over time (20 years), as a function of annual community drug use (p), and the reproductive fitness of resistant parasites. Treatment efficacy and aggregation constants for infection (k) were derived from our study area.
Yearly Efficacy of Praziquantel
During 1984 to 1992, we observed substantial year-to-year variations in cure rates (conversion from egg-positive to egg-negative status on urine Nuclepore filtration examination) for both praziquantel and metrifonate (Table 1). The response to metrifonate treatment declined each year, from 79% in 1984 to 47% in 1987 (p <0.001, Figure 1); in contrast, we observed no consistent downward trend in response to praziquantel treatment, despite repeated use of the drug in many patients (Figure 1 and Figure 2). However, the response to praziquantel varied significantly from year to year (p <0.001), from a cure rate of 96% in 1990 (year 7 of the project) to a cure rate of 65% in 1986 (year 3, p <0.001). This level of efficacy was within the previous range of S. haematobium cure rates, both in Coast Province and elsewhere in Africa (Table 2). In suppression of infection intensity, the praziquantel-mediated reduction of mean S. haematobium egg counts was consistently >83% for all years of observation.
Impact of Repeated Treatment
In both the study periods during which repeated, annual praziquantel treatment was used (1984-1987 and 1989-1993), the observed cure rate for egg-positive children increased in year 2 of treatment, then decreased in year 3 (Figure 1). The number of egg-positive children remaining to be treated in successive years was small, and this sample did not have sufficient statistical power to determine whether the year 3 decrease in praziquantel response was due to fluctuations in transmission (30), progressive selection of patients at high risk for exposure to S. haematobium (27,31), or gradual selection of praziquantel-resistant parasites.
Given the incomplete efficacy of praziquantel in eradicating infection (6) and the single annual follow-up, our study design could not distinguish reinfection from possible drug failure. Therefore, we used several indirect variables to estimate ongoing praziquantel efficacy: the level of persistent egg-positivity at 1 year after praziquantel treatment, compared with rates of new infection (egg-negative to egg-positive conversion) each year; the efficacy of the drug in children receiving a second, third, or fourth dose, compared with efficacy in children receiving a first dose; and the efficacy of the drug in children who remained egg-positive after their first dose compared with efficacy in children whose infections cleared but who subsequently became reinfected.
Persistent Infection vs. Reinfection
The yearly efficacy of praziquantel treatment and infection or reinfection rates (egg-negative to egg-positive conversion) were compiled for the study population (Table 1). Yearly infection or reinfection rates varied from 15% in 1984 to 1985 to a low of 9% in 1985 to 1986 and a high of 21% in 1991 to 1992 (average 13%). Infection rates for children initially treated with praziquantel who subsequently missed 1 year or more of treatment were 22% to 34% in subsequent surveys 2 to 4 years later. These rates suggested a time-dependent average accumulation of reinfection at an annual rate of 9% to 15%. The high transmission levels in 1991 to 1992 (21% new infection or reinfection) may explain the apparent decrease in praziquantel efficacy during this 12-month period. However, estimated transmission did not explain the decreased efficacy seen in 1986 to 1987, when the population egg-negative to -positive conversion rate was only 11%.
Efficacy of Retreatment with Praziquantel
To examine whether an increasing core of resistant infection might account for the higher post-treatment prevalence after year 2 of treatment, we compared the relative annual efficacy in egg-positive patients who received praziquantel for the first time with efficacy in patients receiving their second, third, or fourth treatments. We found that after the first cycle of treatment, i.e., from 1985 on, praziquantel-mediated cure rates did not differ for patients with first-time treatment and those with a history of praziquantel treatment (Figure 2). We further examined the response to second, third, and fourth treatments in patients who did not become egg-negative after their first dose (i.e., initial nonresponse, possibly resistant) (Figure 3). We compared these results with those of patients who tested negative after the first praziquantel dose, then became egg-positive in later years (those reinfected after cure, Figure 3). Children from these two groups, who were at greatest risk for infection with resistant parasites, eventually reverted to egg-negative status after treatment with one to three supplemental praziquantel doses. The two groups did not differ significantly in their response rates to the second, third, or fourth doses.
Without clear evidence of emerging resistance in our treated population, we considered two related questions: Why was resistance not observed, and when might it be expected to emerge under the field conditions tested? To answer these questions, we turned to mathematical models of inheritance of drug resistance.
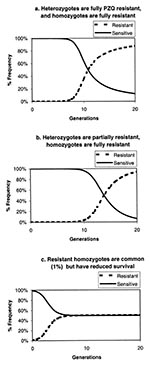
A number of factors may account for the lack of evidence of praziquantel resistance in the Msambweni project. Although S. haematobium resistance to praziquantel is likely to emerge at some future time, the interval required for detection of resistance may be much longer than our 8-year observation period. An effective praziquantel-resistance mutation may be so rare that the number of generations required for it to become the dominant phenotype have not yet occurred. In Egypt, for example, apparently resistant strains of S. mansoni are emerging >10 years after widespread availability of praziquantel treatment (32). For a single resistance gene mutation beginning at frequency of 10-6, a Hardy-Weinberg equilibrium analysis (28) predicts it would take eight or more generations for the resistant phenotype to become clinically detectable (i.e., 25% to 50% of worms) at the community treatment rates used in this study, if the resistance gene heterozygotes are fully resistant to treatment (i.e., a dominant trait [Figure 4a]). In our study, we estimate that 25% to 50% of infections were treated, but because the targeted school-age groups have the highest numbers of worms, 50% to 75% of worms may have been exposed to praziquantel. Emergence times for the resistance phenotype are longer if heterozygotes remain fully or partially susceptible to the drug (11 generations for 25% heterozygote loss after treatment and 14 to 18 generations for 50% heterozygote losses) and only the homozygotes are fully resistant (Figure 4b). Given the seasonal nature of rainfall and water exposure, the requirement of two sexes for schistosome reproduction, and the uneven aggregation of worms in the human population, the effective generation time for S. haematobium is likely to be at least 6 to 12 months, so we observed no more than 8 to 16 generations; our observation period may have been too short to identify praziquantel resistance. Our annual follow-up was an insensitive means to detect drug failure due to resistance. However, under the pressure of continued praziquantel treatment in the community, if resistant worms are fully fit, rapid predominance of resistant strains would be expected (Figure 4).
Praziquantel failure on initial treatment in S. mansoni- endemic areas of Senegal suggests primary resistance to praziquantel (16,18) and a high prevalence of resistance genes in the local S. mansoni strain (21,33,34). More recent reports indicate, however, that in this area of Senegal, retreatment after 40 days adequately reduces infection levels and achieves better cure rates (19). The latter results suggest that immature schistosomes, which are known not to be susceptible to praziquantel, are typically present in Senegalese patients at the time of treatment. If the treated patient has been recently exposed to infection (<6 weeks ago) in an area with continuous--rather than seasonal--transmission, apparent treatment failure may be observed when unaffected juvenile worms reach maturity and pass eggs several weeks after praziquantel treatment (14). Annual reinfection rates for S. haematobium may be high in some areas, such as Niger (35), and post-treatment infection detected after a single round of therapy should not be immediately interpreted as evidence of praziquantel resistance.
Nevertheless, animal studies of S. mansoni strains from Senegal indicate they are less sensitive than strains from other parts of the world (21,33,34). There is another explanation for an apparent low-level prevalence of drug resistance that fails to predominate in the parasite population: if a praziquantel-resistance mutation compromises reproductive fitness, the homozygous, fully resistant worm will never predominate. Instead, under the pressure of continued treatment, the resistance gene will achieve a stable-equilibrium share of the worm population, at a level dependent on its lower survival efficiency relative to that of the praziquantel-sensitive genotype (Figure 4c). This situation is analogous to the effect of the sickle cell-hemoglobin gene in human populations exposed to malaria.
Another possible factor likely to be slowing the emergence of praziquantel resistance in Schistosoma is the parasite's obligate dioecious sexual reproduction. Unlike drug-resistant bacteria, praziquantel-resistant schistosomes must find a mate of the opposite sex to reproduce, which requires a sufficient density of human infection. In a disease-endemic area where most of the population has been treated, the initial heavy loss of susceptible worms (>80%) may actually reduce mean number of worms sufficiently (i.e., to fewer than one male and one female per host) to prevent most resistant worms from finding suitable mates (29). Worm distribution is highly aggregated in the human population, with most (75%) patients having light infections and a small proportion (approximately 5%) having heavy infections. Heavy infection can result in reduced worm fecundity, slowing the production of eggs and thus the transmission of genes from a resistant worm, even though the chances of mating are enhanced. After treatment, the reduced number of worms in a heavily infected human could increase fecundity, so the net effect of treatment on praziquantel-resistance gene transmission would be difficult to predict. In an age-targeted program such as ours, praziquantel-sensitive parasites would also persist in the untreated adult and infant human subpopulations, slowing the dominance of drug resistance gene(s). This would occur by allowing interbreeding of resistant and susceptible worms in host locations not having the environmental pressure (i.e., praziquantel treatment) that favors the resistance gene.
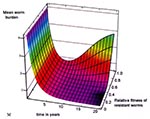
Using a dynamic model of parasite transmission that accounts for these reproductive features of macroparasite transmission (Appendix ), we examined the impact of treatment coverage and duration of control programs on the emergence of resistant infection, to estimate the time required for drug failure. We also examined the impact of varying reproductive fitness of the resistant worms on the time to recrudescence of infection. Figure 5 and Figure 6 show the predicted recrudescence of infection over a 20-year period, as influenced by treatment coverage and by reproductive fitness of resistant worms. Both simulations indicate at least an 8- to 10-year delay in the return of infection to pretreatment levels. This delay is especially noted if treatment coverage is incomplete (Figure 5) and resistant parasites are less reproductively fit than susceptible ones (Figure 6). The model further indicates that under the conditions of our program (targeted mass treatment, with 25% to 75% of infections praziquantel-treated), resistance becomes clinically apparent (>50% resistant worms) only after a period years longer than that we were able to observe.
In our model, we assumed an effective spontaneous mutation rate for praziquantel resistance of 10-6. Recent work by Moxon and colleagues (36-38) on the evolution of bacterial pathogens indicates that genetic mutation becomes accelerated (i.e., nonrandom) in a subset of organisms under environmental stress, such as drug pressure. The "hypermutable" phenotype prevails under these rapidly changing conditions because of its ability to adapt quickly to the new environment. Rapid genetic diversification is enhanced by simultaneous modular changes in DNA, which are facilitated by the presence of multiple copies of critical genes, transposons, and repeat regions or Z-DNA boundaries flanking key intron/exon boundaries (39). Higher organisms, such as schistosomes, are likely to use similar mechanisms in adaptation. Drake (40) has estimated that the effective spontaneous mutation rate per genome per sexual generation may be as high as 10-2 for the nematode C. elegans and 10-1 for the insect Drosophila. In sensitivity analysis of our differential model, reducing the mutation rate from 10-6 to 10-1 in our base case (50% coverage with 85% efficacy) reduced the expected time to emergence of resistance from 10 to 7 years. At higher levels of drug use (90%-100% coverage), the time to resistance was reduced from 7 to 5 years at the highest mutation rate (10-1). This effect is not as dramatic as would be predicted by the simpler Hardy-Weinberg model (i.e., a reduction from 14 to 3 generations for dominance of resistance), apparently because of the retarding effects of aggregation and mating on emergence of new genotypes.
In summary, our results indicate that significant resistance to praziquantel treatment had not emerged in S. haematobium in the Msambweni area as of 1991. As noted since its first use, praziquantel treatment was not 100% effective in eliminating infection (41). It was, however, reliably capable of suppressing intensity of infection in each of six rounds of treatment spaced over 8 years. These findings, together with our modeling analysis, support targeted use of chemotherapy for disease control in S. haematobium-endemic populations (1,26,42-44). In our program, praziquantel treatment was focused only on schoolchildren, the subpopulation having the highest numbers of worms (26,27). Higher treatment coverage increases evolutionary pressure on the schistosome population and hastens emergence of resistance. The limited coverage of a targeted (vs. mass-treatment) program may have reduced the tendency for resistance to emerge, compared with the experience reported for S. mansoni in Egypt (32).
For the treated Msambweni population, the benefits of treatment appeared to be maximal after 2 years of repeated yearly treatment (26,27). After this, a 2- to 3-year interval may be sufficient to maintain effective suppression of infection and disease (27). Because reduced exposure to the drug slows the emergence of drug resistance, the efficacy of longer intertreatment intervals in suppressing S. haematobium-related disease should be studied. Although efficacy should be maintained for a number of years, regular monitoring of drug efficacy is appropriate for all long-term praziquantel-based control programs (14). Emergence of praziquantel resistance should be anticipated within 10 to 20 years, and continued antischistosomal drug development should be pursued.
Dr. King is an associate professor of Medicine and International Health at Case Western Reserve University in Cleveland, Ohio. His current research focuses on modeling of transmission of infectious diseases and prevention of disease due to helminthic infection.
Acknowledgments
We thank the staff of the Division of Vector Borne Diseases, Ministry of Health, Kenya, and the students, residents, and faculty of Case Western Reserve University who worked to make the Msambweni Study a success; the people of the Msambweni area of Kwale District for their enthusiastic participation; and Daren Austin for his description and helpful discussions of macroparasite resistance models.
This work was supported by grants from the Edna McConnell Clark Foundation, the WHO-TDR/UNDP Research Training Program, and the National Institutes of Health (AI 33061, AI45473).
References
- World Health Organization. The control of schistosomiasis: second report of the WHO Expert Committee. Vol. 830 Geneva: 1993 WHO Technical Report.
- Hotez PJ, Zheng F, Long-qi X, Ming-gang C, Shu-hua X, Shu-xian L, Emerging and reemerging helminthiases and the public health of China. Emerg Infect Dis. 1997;3:303–10. DOIPubMedGoogle Scholar
- Praziquantel. USP Drug Information. Vol. I. Rockville (MD): United States Pharmacopeial Convention; 1998:2395-7.
- King CH, Mahmoud AA. Drugs five years later: praziquantel. Ann Intern Med. 1989;110:290–6.PubMedGoogle Scholar
- Davis A, Wegner DH. Multicentre trials of praziquantel in human schistosomiasis: design and techniques. Bull World Health Organ. 1979;57:767–71.PubMedGoogle Scholar
- Brindley PJ. Drug resistance to schistosomicides and other anthelmintics of medical significance. Acta Trop. 1994;56:213–31. DOIPubMedGoogle Scholar
- Boisier P, Ramarokoto CE, Ravaoalimalala VE, Rabarijaona L, Serieye J, Roux J, Reversibility of Schistosoma mansoni-associated morbidity after yearly mass praziquantel therapy: ultrasonographic assessment. Trans R Soc Trop Med Hyg. 1998;92:451–3. DOIPubMedGoogle Scholar
- Chan MS, Nsowah-Nuamah NNN, Adjei S, Wen S, Hall A, Bundy DAP. Predicting impact of school-based treatment for urinary schistosomiasis given by the Ghana Partnership for Child Development. Trans R Soc Trop Med Hyg. 1998;92:386–9. DOIPubMedGoogle Scholar
- Jongsuksuntigul P, Imsomboon T. The impact of a decade long opisthorchiasis control program in northeastern Thailand. Southeast Asian J Trop Med Public Health. 1997;28:551–7.PubMedGoogle Scholar
- Schelling U, Frank W, Will R, Romig T, Lucius R. Chemotherapy with praziquantel has the potential to reduce the prevalence of Echinococcus multilocularis in wild foxes (Vulpes vulpes). Ann Trop Med Parasitol. 1997;91:179–86.PubMedGoogle Scholar
- Llayd S, Walters TM. Worming of dogs in mid-Wales for Echinococcus granulosus. Vet Rec. 1997;140:487–8.PubMedGoogle Scholar
- Olveda RM, Daniel BL, Ramirez BD, Aligui GD, Acosta LP, Fevidal P, Schistosomiasis japonica in the Philippines: the long-term impact of population-based chemotherapy on infection, transmission, and morbidity. J Infect Dis. 1996;174:163–72.PubMedGoogle Scholar
- Renganathan E, Cioli D. An international initiative on praziquantel use. Parasitol Today. 1998;14:390–1. DOIPubMedGoogle Scholar
- Kusel J, Hagan P. Praziquantel--its use, cost and possible development of resistance [news]. Parasitol Today. 1999;15:352–4. DOIPubMedGoogle Scholar
- Guisse F, Polman K, Stelma FF, Mbaye A, Talla I, Niang M, Therapeutic evaluation of two different dose regimens of praziquantel in a recent Schistosoma mansoni focus in Northern Senegal. Am J Trop Med Hyg. 1997;56:511–4.PubMedGoogle Scholar
- Stelma FF, Sall S, Daff B, Sow S, Niang M, Gryseels B. Oxamniquine cures Schistosoma mansoni infection in a focus in which cure rates with praziquantel are unusually low. J Infect Dis. 1997;176:304–7. DOIPubMedGoogle Scholar
- Stelma FF, Talla I, Sow S, Kongs A, Niang M, Polman K, Efficacy and side effects of praziquantel in an epidemic focus of Schistosoma mansoni. Am J Trop Med Hyg. 1995;53:167–70.PubMedGoogle Scholar
- Picquet M, Vercruysse J, Shaw DJ, Diop M, Ly A. Efficacy of praziquantel against Schistosoma mansoni in northern Senegal. Trans R Soc Trop Med Hyg. 1998;92:90–3. DOIPubMedGoogle Scholar
- Bennett JL, Day T, Feng-Tao L, Ismail M, Farghaly A. The development of resistance to anthelmintics: A perspective with an emphasis on the antischistosomal drug praziquantel. Exp Parasitol. 1997;87:260–7. DOIPubMedGoogle Scholar
- Fallon PG, Mubarak JS, Fookes RE, Niang M, Butterworth AE, Sturrock RF, Schistosoma mansoni: maturation rate and drug susceptibility of different geographic isolates. Exp Parasitol. 1997;86:29–36. DOIPubMedGoogle Scholar
- Pereira C, Fallon PG, Cornette J, Capron A, Doenhoff MJ, Pierce RJ. Alterations in cytochrome-c oxidase expression between praziquantel-resistant and susceptible strains of Schistosoma mansoni. Parasitology. 1998;117:63–73. DOIPubMedGoogle Scholar
- Cunha VM, Noel F. (Ca(2+)-Mg2+)ATPase in Schistosoma mansoni: evidence for heterogeneity and resistance to praziquantel. Mem Inst Oswaldo Cruz. 1998;93:181–2. DOIPubMedGoogle Scholar
- Cioli D, Pica Mattoccia L. Genetic analysis of hycanthone resistance in Schistosoma mansoni. Am J Trop Med Hyg. 1984;33:80–8.PubMedGoogle Scholar
- King CH, Lombardi G, Lombardi C, Greenblatt R, Hodder S, Kinyanjui H, Chemotherapy-based control of schistosomiasis haematobia. I. Metrifonate versus praziquantel in control of intensity and prevalence of infection. Am J Trop Med Hyg. 1988;39:295–305.PubMedGoogle Scholar
- King CH, Muchiri EM, Ouma JH. Age-targeted chemotherapy for control of urinary schistosomiasis in endemic populations. Mem Inst Oswaldo Cruz. 1992;87:203–10. DOIPubMedGoogle Scholar
- Muchiri EM, Ouma JH, King CH. Dynamics and control of Schistosoma haematobium transmission in Kenya: an overview of the Msambweni Project. Am J Trop Med Hyg. 1996;55:127–34.PubMedGoogle Scholar
- Khoury MJ, Beaty TH, Cohen BH. Fundamentals of genetic epidemiology. New York: Oxford University Press; 1993.
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. New York: Oxford University Press; 1991. pp. 467-96; 507-20.
- Sturrock RF, Kinyanjui H, Thiongo FW, Tosha S, Ouma JH, King CH, Chemotherapy-based control of schistosomiasis haematobia. 3. Snail studies monitoring the effect of chemotherapy on transmission in the Msambweni area, Kenya. Trans R Soc Trop Med Hyg. 1990;84:257–61. DOIPubMedGoogle Scholar
- Muchiri EM. Association of water contact activities and risk of reinfection for S. haematobium after drug treatment in the Msambweni Area, Kenya [MS thesis, Epidemiology and Biostatistics]. Cleveland (OH): Case Western Reserve University; 1991.
- Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennett JL. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg. 1996;55:214–8.PubMedGoogle Scholar
- Fallon PG, Sturrock RF, Niang AC, Doenhoff MJ. Short report: diminished susceptibility to praziquantel in a Senegal isolate of Schistosoma mansoni. Am J Trop Med Hyg. 1995;53:61–2.PubMedGoogle Scholar
- Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg. 1994;51:83–8.PubMedGoogle Scholar
- Laurent C, Lamothe F, Develoux M, Sellin B, Mouchet F. Ultrasonographic assessment of urinary tract lesions due to Schistosoma haematobium in Niger after four consecutive years of treatment with praziquantel. Trop Med Parasitol. 1990;41:139–42.PubMedGoogle Scholar
- Rainey BP, Moxon ER. Microbiology. When being hyper keeps you fit. Science. 2000;288:1186–7. DOIPubMedGoogle Scholar
- Field D, Magnasco MO, Moxon ER, Metzgar D, Tanaka MM, Wills C, Contingency loci, mutator alleles, and their interactions. Synergistic strategies for microbial evolution and adaptation in pathogenesis. Ann N Y Acad Sci. 1999;870:378–82. DOIPubMedGoogle Scholar
- Moxon ER, Rainey PB, Nowak MA, Lenski RE. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol. 1994;4:24–33. DOIPubMedGoogle Scholar
- Caporale LH. Chance favors the prepared genome. Ann N Y Acad Sci. 1999;870:1–21. DOIPubMedGoogle Scholar
- Drake JW. The distribution of rates of spontaneous mutation over viruses, prokaryotes, and eukaryotes. Ann N Y Acad Sci. 1999;870:100–7. DOIPubMedGoogle Scholar
- Davis A, Biles JE, Ulrich AM. Initial experiences with praziquantel in the treatment of human infections due to Schistosoma haematobium. Bull World Health Organ. 1979;57:773–9.PubMedGoogle Scholar
- Mott KE, Dixon H, Osei-Tutu E, England EC, Davis A. Effect of praziquantel on hematuria and proteinuria in urinary schistosomiasis. Am J Trop Med Hyg. 1985;34:1119–26.PubMedGoogle Scholar
- Stephenson LS, Latham MC, Kinoti SN, Oduori ML. Sensitivity and specificity of reagent strips in screening of Kenyan children for Schistosoma haematobium infection. Am J Trop Med Hyg. 1984;33:862–71.PubMedGoogle Scholar
- Pugh RN, Bell DR, Gilles HM. Malumfashi Endemic Diseases Research Project, XV. The potential medical importance of bilharzia in northern Nigeria: a suggested rapid, cheap and effective solution for control of Schistosoma haematobium infection. Ann Trop Med Parasitol. 1980;74:597–613.PubMedGoogle Scholar
- Olds GR, King CH, Hewlett J, Olveda R, Wu G, Ouma JH, Double-blind placebo controlled study of concurrent administration of albendazole and praziquantel in school children with schistosomiasis and geohelminths. J Infect Dis. 1999;179:996–1003. DOIPubMedGoogle Scholar
- King CH, Wiper DWD, De Stigter KV, Peters PA, Koech D, Ouma JH, . Dose-finding study for praziquantel therapy of Schistosoma haematobium in Coast Province, Kenya. Am J Trop Med Hyg. 1989;40:507–13.PubMedGoogle Scholar
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN. Single dose metrifonate or praziquantel treatment in Kenyan children. II. Effects on growth in relation to Schistosoma haematobium and hookworm egg counts. Am J Trop Med Hyg. 1989;41:445–53.PubMedGoogle Scholar
- Shaw DJ, Vercruysse J, Picquet M, Sambou B, Ly A. The effect of different treatment regimens on the epidemiology of seasonally transmitted Schistosoma haematobium infections in four villages in the Senegal River Basin, Senegal. Trans R Soc Trop Med Hyg. 1999;93:142–50. DOIPubMedGoogle Scholar
- Taylor P, Murare HM, Manomano K. Efficacy of low doses of praziquantel for Schistosoma mansoni and S. haematobium. J Trop Med Hyg. 1988;91:13–7.PubMedGoogle Scholar
- Etard JF, Borel E, Segala C. Schistosoma haematobium infection in Mauritania: two years of follow-up after a targeted chemotherapy--a life-table approach of the risk of reinfection. Parasitology. 1990;100:399–406. DOIPubMedGoogle Scholar
- el Malatawy A, el Habashy A, Lechine N, Dixon H, Davis A, Mott KE. Selective population chemotherapy among schoolchildren in Beheira governorate: the UNICEF/Arab Republic of Egypt/WHO Schistosomiasis Control Project. Bull World Health Organ. 1992;70:47–56.PubMedGoogle Scholar
- Wilkins HA, Blumenthal UJ, Hayes RJ, Tulloch S. Resistance to reinfection after treatment of urinary schistosomiasis. Trans R Soc Trop Med. 1987;81:29–35. DOIPubMedGoogle Scholar
- McMahon JE, Kolstrup N. Praziquantel: a new schistosomicide against Schistosoma haematobium. BMJ. 1979;2:1396–8. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 6, Number 6—December 2000
| EID Search Options |
|---|
|
|
|
|
|
|