Volume 7, Number 2—April 2001
THEME ISSUE
4th Decennial International Conference on Nosocomial and Healthcare-Associated Infections
State of the Art
Increasing Resistance to Vancomycin and Other Glycopeptides in Staphylococcus aureus
Cite This Article
Citation for Media
Abstract
Strains of Staphylococcus aureus with reduced susceptibility to glycopeptides have been reported from Japan, the United States, Europe, and the Far East. Although isolates with homogeneous resistance to vancomycin (MICs = 8 µg/mL) continue to be rare, there are increasing reports of strains showing heteroresistance, often with vancomycin MICs in the 1-4 µg/mL range. Most isolates with reduced susceptibility to vancomycin appear to have developed from preexisting methicillin-resistant S. aureus infections. Many of the isolates with reduced susceptibility to glycopeptides have been associated with therapeutic failures with vancomycin. Although nosocomial spread of the vancomycin-intermediate S. aureus (VISA) strains has not been observed in U.S. hospitals, spread of VISA strains has apparently occurred in Japan. Broth microdilution tests held a full 24 hours are optimal for detecting resistance in the laboratory; however, methods for detecting heteroresistant strains are still in flux. Disk-diffusion tests, including the Stokes method, do not detect VISA strains. The Centers for Disease Control and Prevention and other groups have issued recommendations regarding appropriate infection control procedures for patients infected with these strains.
Staphylococcus aureus continues to be a major cause of community-acquired and health-care related infections in the United States and around the world (1,2). Approximately 20% of community-acquired and nosocomial bacteremias in the United States are caused by S. aureus (3–5). The emergence of high levels of penicillin resistance followed by the development and spread of strains resistant to the semisynthetic penicillins (methicillin, nafcillin, and oxacillin), macrolides, tetracyclines, and aminoglycosides has made therapy of staphylococcal disease a global challenge (1,6,7). In the 1980s, because of widespread occurrence of methicillin-resistant S. aureus (MRSA), empiric therapy for staphylococcal infections (particularly nosocomial sepsis) was changed to vancomycin in many health-care institutions (8–12). Vancomycin use in the United States also increased during this period because of the growing numbers of infections with Clostridium difficile and coagulase-negative staphylococci in health-care facilities (8,9). Thus, the early 1990s saw a discernible increase in vancomycin use. As a consequence, selective pressure was established that eventually led to the emergence of strains of S. aureus and other species of staphylococci with decreased susceptibility to vancomycin and other glycopeptides.
In 1997, the first strain of S. aureus with reduced susceptibility to vancomycin and teicoplanin was reported from Japan (13). Shortly thereafter, two additional cases from the United States were reported (14). While vancomycin therapy appeared to have failed in the patients infected with these organisms, debate was considerable about whether such strains should be designated as resistant to glycopeptides, since the levels of vancomycin required to inhibit the growth of the strains remained low (vancomycin MIC = 8 µg/mL). Three years later, the debate continues. At the heart of the discussion are conflicting definitions of resistance and resistance breakpoints, a handful of nonstandardized laboratory methods, and a very small sample size of strains collected from the far corners of the world upon which to draw conclusions (15–17). We address this question of reduced susceptibility versus resistance.
The National Committee for Clinical Laboratory Standards (NCCLS) defines staphylococci requiring concentrations of vancomycin of < 4 µg/mL for growth inhibition as susceptible, those requiring 8 µg/mL to 16 µg/mL for inhibition as intermediate, and those requiring concentrations of > 32 µg/mL as resistant (18). Similarly, for teicoplanin (a drug not approved for use in the United States), staphylococci requiring inhibitory concentrations of <8 µg/mL are designated as susceptible, those requiring 16 µg/mL for inhibition as intermediate, and those requiring concentrations of > 32 µg/mL as resistant. Thus, the acronyms VISA (vancomycin-intermediate S. aureus) and GISA (glycopeptide-intermediate S. aureus) come directly from the interpretive criteria published by NCCLS. While GISA is technically a more accurate description of the strains isolated to date, since most are classified as intermediate to both vancomycin and teicoplanin, the term glycopeptide may not be recognized by many clinicians. Thus, the term VISA, which emphasizes a change in vancomycin MICs similar to vancomycin-resistant enterococci (VRE), may be a more effective way of communicating to clinicians the changes occurring in the susceptibility of staphylococci to vancomycin. Although NCCLS has also defined disk-diffusion criteria for interpretation of vancomycin results for staphylococci (19), this method is not sufficiently sensitive to detect decreased susceptibility to vancomycin in staphylococci and should not be used for routine testing of staphylococci (19,20).
In the United States, the term vancomycin-resistant S. aureus (VRSA) is reserved for S. aureus strains for which the vancomycin or teicoplanin MICs are > 32 µg/mL, as is also true in France, where the Comité de l'Antibiogramme de la Société Française Microbiologie has published breakpoints similar to those of NCCLS (21). However, using the interpretive criteria of the British Society for Antimicrobial Chemotherapy, strains for which the vancomycin MICs are > 8 µg/mL would be reported as VRSA (22). Interpretive criteria for vancomycin from these three organizations are shown (Table 1).
The term VRSA also has been used by Japanese investigators to denote strains of S. aureus that grow on a brain heart infusion screening (BHI) agar plate containing 4 µg/mL of vancomycin within 24 hours, provided that the vancomycin broth microdilution MIC is at least 8 µg/mL (23). Those strains that produce colonies on vancomycin-containing BHI agar with vancomycin MICS of < 4 µg/mL are termed heteroresistant VRSA or hetero-VRSA. By population analysis, subpopulations can be detected in hetero-VRSA strains, often representing only 1 in 100,000 daughter cells, for which the vancomycin MICs are 8 µg/mL. Such strains were first reported from Japan in 1996 (13). The prototype strain is S. aureus Mu3, for which the vancomycin MIC range (by standard broth microdilution testing) is 1 µg/mL to 2 µg/mL. Often the vancomycin MICs reported for hetero-VRSA isolates in the literature are those obtained from colonies preselected on vancomycin-containing media and are not those of the original isolate. As Howe et al. point out, this process may, in fact, be selecting for resistance in vitro rather than screening for it (24). Whether the isolation of such hetero-VRSA strains from patients explains the apparent failure of vancomycin therapy remains controversial. While some of the isolates, such as those from Hong Kong (25), have been associated with therapeutic failures with vancomycin, many hetero-VRSA strains (or hetero-VISA strains, as they are also known) were detected through retrospective laboratory screening of MRSA isolates, and the clinical significance of the isolates is unknown (26–28).
Strains of VISA (vancomycin MIC = 8 µg/mL) have been reported from Japan (13), the United States (29–31), France (32), United Kingdom (24), and Germany (26). Most of these isolates appear to have developed from preexisting MRSA infections. Hetero-VRSA strains have been reported from Spain (33), Scotland (34), Hong Kong (25), Germany (26,28), and Greece, among other countries (27). Most of these isolates were detected during retrospective testing surveys using BHI agar containing 4 µg/mL of vancomycin. For example, a hetero-VRSA isolate from Egypt, first isolated in 1981, was not identified until 1998 during a retrospective review of MRSA strains by Bierbaum et al. (26).
Evidence from the few affected U.S. patients investigated to date suggests that infections caused by VISA, for which the vancomycin MICs are 8 µg/mL, are refractory to vancomycin therapy (29). The Centers for Disease Control and Prevention (CDC) has received reports of several other infections caused by S. aureus for which the vancomycin MICs were 4 µg/mL, which suggests that some of these patients did not improve on appropriate vancomycin therapy. Data from rabbit endocarditis models presented by Climo et al. (35) also suggest that vancomycin monotherapy is not adequate for VISA strains. However, the combination of oxacillin and vancomycin is synergistic both in vitro and in vivo in the endocarditis model (35). Similar data on the synergy of beta-lactams and vancomycin for VISA strains were reported by Sieradzki et al. (36). However, the accumulated experience from humans and animals is too small for firm conclusions regarding a loss in the effectiveness of vancomycin for such infections, particularly those caused by strains of S. aureus that are heteroresistant to glycopeptides. Our inability to differentiate in the laboratory between vancomycin-susceptible S. aureus strains (i.e., those for which the vancomycin MICs are < 2 µg/mL) that have vancomycin-resistant subpopulations versus those vancomycin-susceptible strains that do not have such subpopulations hinders our efforts to clarify the effectiveness of vancomycin for staphylococcal infections.
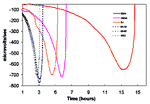
The mechanisms by which S. aureus isolates become more resistant to vancomycin are poorly understood. However, many of the clinical and laboratory-derived strains with decreased susceptibility to vancomycin share unique features. For example, most VISA strains for which the vancomycin MICs are 8 µg/mL show longer doubling times, decreased lysostaphin susceptibilities, and reduced autolytic activity (37,38). Studies conducted at CDC with Mu50 and the Michigan and the New Jersey VISA strains used changes in redox potential over time as an indicator of bacterial growth measured by using a Cytosensor Microphysiometer System (Molecular Devices Corporation, Sunnyvale, CA). These studies showed dramatically longer doubling times for the VISA strains (Figure 1, three curves on right) compared with the methicillin- and vancomycin-susceptible control strain S. aureus ATCC 25923 and two MRSA control strains obtained from CDC (3 curves on left side of Figure 1). However, several authors have noted that the vancomycin MICs for VISA strains are not stable and decrease over time in the absence of selective pressure (35,37,38).
Hanaki et al. reported that hetero-VRSA produced three- to five-fold greater quantities of penicillin-binding proteins 2 and 2' and increased quantities of cell-wall precursors, which presumably trap vancomycin extracellularly (39). In addition, amidation of glutamine residues in cell-wall muropeptides has been reported, which presumably reduces the cross-linking within the cell walls, thereby also reducing the number of intracellular vancomycin target molecules (40). Geisel et al. reported similar biochemical changes in seven MRSA isolates with reduced susceptibility to vancomycin (hetero-VISA) isolated from patients from three hospitals in Düsseldorf, Germany (28). Two of the patients had received vancomycin before the hetero-VISA strains were isolated. All seven isolates, obtained in 1998, had identical pulsed-field gel electrophoresis profiles identical to that of the northern Germany epidemic strain. Whether heteroresistance is a characteristic of all the progeny of this clone is unknown.
Most VISA isolates initially appear mixed, demonstrating two distinct colony types; however, both colony types yield identical antimicrobial susceptibility test results (Figure 2). Decreased susceptibility to vancomycin (i.e., an MIC of vancomycin of 8 µg/mL) was detected in the S. aureus isolates from Michigan and New Jersey by broth microdilution when incubated for 24 hours at 35°C (20). On the other hand, the isolate from New York often demonstrated a vancomycin MIC of 4 µg/mL by broth microdilution but an MIC of 6 µg/mL by Etest methods. Thus, a single MIC test method may not be accurate enough to detect all VISA strains. CDC has adopted three criteria to identify VISA strains (Table 2), broth microdilution vancomycin MICs of 8-16 µg/mL, Etest (AB Biodisk, Piscataway, NJ) vancomycin MICs of > 6 µg/mL, and growth on commercial BHI agar screen plates containing 6 µg/mL of vancomycin within 24 hours.
VISA isolates are not reliably distinguished from vancomycin-susceptible isolates by the rapid automated methods, such as MicroScan (Dade MicroScan, West Sacramento, CA) rapid panels (20). NCCLS disk-diffusion method and the Stokes method are not accurate predictors of reduced vancomycin susceptibility in staphylococci (20,41). Recent changes in Vitek (Biomérieux, Hazelwood, MO) software (version 7.01) may have improved VISA detection (CDC, unpub. obs.).
The clinical significance of heteroresistance is an issue of considerable controversy regarding the emergence of decreased susceptibility of staphylococci to vancomycin. Staphylococcal isolates with vancomycin MICs of 1 µg/mL to 4 µg/mL can be heterogeneous, that is, only small subpopulations of the isolates will grow in the presence of vancomycin concentrations of 8 µg/mL to 16 µg/mL, often 1 daughter cell in 105 CFU. Identifying isolates with subpopulations demonstrating heterogeneous resistance to vancomycin is difficult. CDC has chosen to use an inoculum of 106 CFU/mL on BHI containing 6 µg/mL of vancomycin for screening. All the isolates for which the vancomycin MICs are 8 µg/mL grow on these screening plates. Mu3, the hetero-VRSA strain from Japan, does not grow on this medium (20). Hiramatsu et al. (23) suggest using an inoculum of 108 CFU/mL on BHI agar containing 4 µg/mL of vancomycin and cell-wall precursors (called Mu3 supplement) to screen for hetero-VRSA. Others have used this approach, omitting the supplements (26). Bierbaum et al. reported that 23 of 25 isolates showing growth on BHI agar containing 4 µg/ml of vancomycin were classified as susceptible by NCCLS criteria (vancomycin MICs 4 µg/mL) even after growth on agar containing < 4 µg/mL vancomycin. For the remaining two isolates, the vancomycin MICs were 8 µg/mL; however, the inoculum for the test was taken from vancomycin-containing agar. In our experience (42), growth of a variety of S. aureus isolates on screening plates with concentrations of 4 µg/mL of vancomycin is not unusual, but rarely do such strains have elevated vancomycin MICs. Thus, the clinical significance of such isolates remains unclear. Until further clinical data are available to assess the significance of heteroresistance, routine screening of S. aureus isolates for vancomycin-heteroresistant subpopulations is not warranted in the United States. Such screening may be undertaken as part of research protocols, but results generated using screening agars with low concentrations of vancomycin, the Etest method with a high inoculum (108 CFU/mL) on BHI agar with prolonged incubation, or vancomycin high-salt agar should not be reported as VRSA on a patient's medical record.
A recent survey of laboratories participating in CDC's Emerging Infections Program indicated that many are not using methods that can detect VISA strains (43). Yet, it is crucial that laboratories develop an algorithm for identifying VISA in their institutions if our understanding of how to treat these infections is to improve. Screening all isolates of S. aureus is neither cost-effective nor prudent at this time, given the low prevalence of such strains. Rather, focusing screening efforts on MRSA isolates is likely to be more successful since most VISA and hetero-VRSA isolates to date have been MRSA. With regard to surveillance of patient populations, hemodialysis and chronic ambulatory peritoneal dialysis patients are known to be at high risk for developing MRSA infections since they frequently are carriers of MRSA (44) and often receive long-term glycopeptide therapy. Such patients may be monitored for emerging VISA infections as should other patients who are predisposed to MRSA infections and receive vancomycin.
The most prudent approach to curtailing the spread of VISA infections is still a matter of opinion. CDC has issued interim guidelines to aid hospitals in establishing programs for control of staphylococci with reduced susceptibility to vancomycin (45), and CDC's Hospital Infection Control Practices Advisory Committee has published guidelines for prudent vancomycin use (46). Others have suggested alternative approaches (47). The transfer of VISA strains beyond the source patient has not been documented in the United States, perhaps because the patients reported in Michigan and New Jersey were already in isolation because of pre-existing MRSA or vancomycin-resistant enterococcal infections (29). Identification of a VISA infection in a health-care setting should prompt a careful epidemiologic investigation. Since MRSA are known to be highly transmissible in health-care settings, it is reasonable to assume that VISA isolates would be no less transmissible given the opportunity.
The antibiograms of U.S., German, and French VISA isolates (Table 3) show that isolates remained susceptible to at least some common antimicrobial agents, such as trimethoprim-sulfamethoxazole, as well as to newer agents, such as linezolid and quinupristin-dalfopristin (20). However, the possibility that newer VISA isolates will be resistant to all common drugs in addition to glycopeptides has to be considered. Several of the patients with VISA isolates from Japan and the United States responded to alternate therapies that included arbikacin and ampicillin-sulbactam, gentamicin, and trimethoprim-sulfamethoxazole. Whether the next VISA isolate will have a more resistant antibiogram is a matter of considerable speculation.
To date, staphylococci harboring the vancomycin resistance genes from enterococci have not been isolated from clinical samples, although some investigators have specifically looked for them (20,38,48). However, isolates of staphylococci appear to have achieved clinically relevant levels of resistance that lead to treatment failures even without the vancomycin resistance genes from enterococci. While CDC recommends that enhanced infection control efforts be initiated for S. aureus isolates for which the vancomycin MICs are 8 µg/mL (45), the need for such precautions for strains with MICs of 4 µg/mL is under debate. Such strains of staphylococci, including species other than S. aureus (49,50), will continue to emerge, particularly in patients who receive long-term vancomycin therapy. Thus, efforts to contain VISA infections before they become truly resistant to all available antimicrobial agents should be an infection control priority.
Dr. Tenover is associate director for laboratory science, Division of Healthcare Quality Promotion in the National Center for Infectious Diseases, CDC. His research interests include the molecular basis of antimicrobial-drug resistance in bacteria and development and implementation of bacterial strain typing strategies. Dr. Tenover is also director of the World Health Organization's Collaborating Centre on Global Monitoring of Bacterial Resistance to Antimicrobial Agents.
References
- Kauffman CA, Bradley SF. Epidemiology of community-acquired infection. In: Crossley KB, Archer GL, editors. The staphylococci in human disease. New York: Churchill Livingstone; 1997. p. 287-308.
- Cockerill FR III, Hughes JG, Vetter EA, Mueller RA, Weaver AL, Ilstrup DM, Analysis of 281,797 consecutive blood cultures performed over an eight-year period: trends in microorganisms isolated and the value of anaerobic culture of blood. Clin Infect Dis. 1997;24:403–18. DOIPubMedGoogle Scholar
- Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G, The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602.PubMedGoogle Scholar
- Vallés J, León C, Alvarez-Lerma F. Nosocomial bacteremia in critically ill patients: a multicenter study evaluating epidemiology and prognosis. Clin Infect Dis. 1997;24:387–95. DOIPubMedGoogle Scholar
- Struelens MJ, Mertens R. the Groupement pour le Dépistage, l'Etude et la Prévention des Infections Hospitalières. National survey of methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1994;13:56–63. DOIPubMedGoogle Scholar
- Maranan MC, Moreira B, Boyle-Vavra S, Daum RS. Antimicrobial resistance in staphylococci: epidemiology, molecular mechanisms, and clinical relevance. Infect Dis Clin North Am. 1997;11:813–49. DOIPubMedGoogle Scholar
- Ena J, Dick RW, Jones RN, Wenzel RP. The epidemiology of intravenous vancomycin usage in a university hospital: a 10 year study. JAMA. 1993;269:598–602. DOIPubMedGoogle Scholar
- Kernodle DS, Kaiser AB. Postoperative infections and antimicrobial prophylaxis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone; 1996. p. 2742-56.
- Kirst HA, Thompson DG, Nicas TI. Historical yearly usage of vancomycin. Antimicrob Agents Chemother. 1998;42:1303–4.PubMedGoogle Scholar
- Fridkin SK, Edwards JR, Pichette SC, Pryor ER, McGowan JE Jr, Tenover FC, Determinants of vancomycin use in adult intensive care units in 41 United States Hospitals. Clin Infect Dis. 1999;28:1119–25. DOIPubMedGoogle Scholar
- Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–6. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin-United States, 1997. MMWR Morb Mortal Wkly Rep. 1997;46:765–6.PubMedGoogle Scholar
- Tenover FC. VRSA, VISA, and GISA: the dilemma behind the name game. Clin Microbiol Newsl. 2000;22:49–53. DOIGoogle Scholar
- Johnson AP. Intermediate vancomycin resistance in Staphylococcus aureus: a major threat or a minor inconvenience? J Antimicrob Chemother. 1998;42:289–91. DOIPubMedGoogle Scholar
- Waldvogel FA. New resistance in Staphylococcus aureus. N Engl J Med. 1999;340:556–7. DOIPubMedGoogle Scholar
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 5th ed. Approved standard M7-A5. Wayne (PA): The Committee; 2000.
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. 7th ed. Approved standard M2-A7. Wayne (PA): The Committee; 2000.
- Tenover FC, Lancaster MV, Hill BC, Steward C, Stocker S, Hancock G, Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J Clin Microbiol. 1998;36:1020–7.PubMedGoogle Scholar
- Goldstein F, Soussy C-J, Thabaut A. Report of the Comité de l'Antibiogramme de la Société Française de Microbiologie. Definition of the clinical antibacterial spectrum of activity. Clin Microbiol Infect. 1996;2:S40–9. DOIPubMedGoogle Scholar
- Working Party of the British Society for Antimicrobial Chemotherapy. Breakpoints in in-vitro antibiotic susceptibility testing. J Antimicrob Chemother. 1988;21:701–10. DOIPubMedGoogle Scholar
- Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–3. DOIPubMedGoogle Scholar
- Howe RA, Bowker KE, Walsh TR, Feest TG, MacGowan AP. Vancomycin-resistant Staphylococcus aureus. Lancet. 1998;351:602. DOIPubMedGoogle Scholar
- Wong SS, Ho PL, Woo PC, Yuen KY. Bacteremia caused by staphylococci with inducible vancomycin heteroresistance. Clin Infect Dis. 1999;29:760–7. DOIPubMedGoogle Scholar
- Bierbaum G, Fuchs K, Lenz W, Szekat C, Sahl H-G. Presence of Staphylococcus aureus with reduced susceptibility to vancomycin in Germany. Eur J Clin Microbiol Infect Dis. 1999;18:691–6. DOIPubMedGoogle Scholar
- Kantzanou M, Tassios PT, Tseleni-Kotsovili A, Legakis NJ, Vatopoulos AC. Reduced susceptibility to vancomycin of nosocomial isolates of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1999;43:729–31. DOIPubMedGoogle Scholar
- Geisel R, Schmitz F-J, Thomas L, Berns G, Zetsche O, Ulrich B, Emergence of heterogeneous intermediate vancomycin resistance in Staphylococcus aureus isolates in the Düsseldorf area. J Antimicrob Chemother. 1999;43:846–8. DOIPubMedGoogle Scholar
- Smith T, Pearson ML, Wilcox KR, Cruz C, Lancaster ML, Robinson-Dunn B, Emergence of vancomycin resistance in Staphylococcus aureus: epidemiology and clinical significance. N Engl J Med. 1999;340:493–501. DOIPubMedGoogle Scholar
- Rotun SS, McMath V, Schoonmaker DJ, Maupin PS, Tenover FC, Hill BC, Staphylococcus aureus with reduced susceptibility to vancomycin isolated from a patient with fatal bacteremia. Emerg Infect Dis. 1999;5:147–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Staphylococcus aureus with reduced susceptibility to vancomycin-Illinois, 1999. MMWR Morb Mortal Wkly Rep. 2000;48:1165–7.PubMedGoogle Scholar
- Ploy MC, Grélaud C, Martin C, de Lumley L, Denis F. First clinical isolate of vancomycin-intermediate Staphylococcus aureus in a French hospital. Lancet. 1998;351:1212. DOIPubMedGoogle Scholar
- Ariza J, Pujol M, Cabo J, Pena C, Fernandez N, Linares J, Vancomycin in surgical infections due to methicillin-resistant Staphylococcus aureus with heterogeneous resistance to vancomycin. Lancet. 1999;353:1587–8. DOIPubMedGoogle Scholar
- Hood J, Cosgrove B, Curran E, Lockhart M, Thakker B, Gemmell C, Vancomycin-intermediate resistant Staphylococcus aureus in Scotland. Abstracts of the 4th Decennial International Conference on Nosocomial and HealthCare-Associated Infections, Mar 2000, Atlanta, Georgia. Atlanta: Centers for Disease Control and Prevention; 2000.
- Climo MW, Patron RL, Archer GL. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibility to vancomycin. Antimicrob Agents Chemother. 1999;43:1747–53.PubMedGoogle Scholar
- Sieradzki K, Roberts RB, Haber SW, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–23. DOIPubMedGoogle Scholar
- Pfeltz RF, Singh VK, Schmidt JL, Batten MA, Baranyk CS, Nadakavukaren MJ, Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob Agents Chemother. 2000;44:294–303. DOIPubMedGoogle Scholar
- Boyle-Vavra S, Berke SK, Lee JC, Daum RS. Reversion of glycopeptide resistance phenotype in Staphylococcus aureus clinical isolates. Antimicrob Agents Chemother. 2000;44:272–7. DOIPubMedGoogle Scholar
- Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, Daum RS, Labischinski H, Hiramatsu K. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother. 1998;42:199–209. DOIPubMedGoogle Scholar
- Hanaki H, Labischinski H, Inaba Y, Kondo N, Murakami H, Hiramatsu K. Increase in glutamine-non-amidated muropeptides in the peptidoglycan of vancomycin-resistant Staphylococcus aureus strain Mu50. J Antimicrob Chemother. 1998;42:315–20. DOIPubMedGoogle Scholar
- Fitch L, Johnson AP. Reduced susceptibility to teicoplanin in a methicillin-resistant strain of Staphylococcus aureus. J Antimicrob Chemother. 1998;41:578. DOIPubMedGoogle Scholar
- Hubert SK, Mohammed JM, Fridkin SK, Gaynes RP, McGowan JE Jr, Tenover FC. Glycopeptide-intermediate Staphylococcus aureus: evaluation of a novel screening method and results of a survey of selected U.S. Hospitals. J Clin Microbiol. 1999;37:3590–3.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Laboratory capacity to detect antimicrobial resistance, 1998. MMWR Morb Mortal Wkly Rep. 2000;48:1167–71.PubMedGoogle Scholar
- Zimakoff J, Pedersen FB, Bergen L, Baagø-Nielsen J, Daldorph B, Espersens F, Staphylococcus aureus carriage and infections among patients in four haemo- and peritoneal-dialysis center in Denmark. J Hosp Infect. 1996;33:289–300. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Interim guideline for prevention and control of staphylococcal infection associated with reduced susceptibility to vancomycin. MMWR Morb Mortal Wkly Rep. 1997;46:626–8, 635–6.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Recommendations for preventing the spread of vancomycin resistance: recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Morb Mortal Wkly Rep MMWR 1995;44 (no. RR-12).
- Edmonds MB, Wenzel RP, Pasculle AW. Vancomycin-resistant Staphylococcus aureus: perspectives on measures needed for control. Ann Intern Med. 1996;124:329–34.PubMedGoogle Scholar
- Franchi D, Climo MW, Wong AHM, Edmond MB, Wenzel RP. Seeking vancomycin resistant Staphylococcus aureus among patients with vancomycin-resistant enterococci. Clin Infect Dis. 1999;29:1556–8. DOIPubMedGoogle Scholar
- Sieradzki K, Roberts R, Serur D, Hargrave J, Tomasz A. Heterogeneously vancomycin-resistant Staphylococcus epidermidis strain causing recurrent peritonitis in a dialysis patient during vancomycin therapy. J Clin Microbiol. 1999;37:39–44.PubMedGoogle Scholar
- Pagano L, Tacconelli E, Tumbarello M, Laurenti L, Mele L, Spanu T, Teicoplanin-resistant coagulase-negative staphylococcal bacteraemia in patients with haemotologic malignancies: a problem of increasing importance. J Antimicrob Chemother. 1997;40:738–40. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 7, Number 2—April 2001
| EID Search Options |
|---|
|
|
|
|
|
|