Volume 7, Number 3—June 2001
Dispatch
Outbreak of Influenza in Highly Vaccinated Crew of U.S. Navy Ship
Cite This Article
Citation for Media
Abstract
An outbreak of influenza A (H3N2) occurred aboard a U.S. Navy ship in February 1996, despite 95% of the crew's having been appropriately vaccinated. Virus isolated from ill crew members was antigenicly distinct from the vaccination strain. With an attack rate of 42%, this outbreak demonstrates the potential for rapid spread of influenza in a confined population and the impact subsequent illness may have upon the workplace.
Influenza is a highly contagious virus transmitted by the respiratory route by inhalation of aerosols as well as by direct contact with animate or inanimate objects (1). Because it can spread rapidly in persons in semiclosed or crowded environments, influenza epidemics have posed major risks to military populations (2). The impact of influenza on the U.S. Navy and Marine Corps has been greatly reduced since an annual immunization program began in 1954 (3,4). This mandatory program uses the influenza vaccine reformulated annually by the Vaccine and Related Biological Products Advisory Committee, Food and Drug Administration (5). The 1995/1996 influenza vaccination program contained A/Texas/36/91(H1N1), A/Johannesburg/ 33/94(H3N2), and B/Harbin/7/94 (6).
Data from the Centers for Disease Control and Prevention (CDC) indicate that the earliest specimen collection date for a new variant, A/Wuhan/359/95(H3N2), was July 1995. The variant was later identified in China (during August, September, and October 1995), Hong Kong (October 1995), and Guam (November 1995). During the winter months of 1996, A/Wuhan/359/95-like viruses were isolated in Asia, Europe, and North America (7). In February 1996, this virus caused an outbreak onboard a U.S. Navy ship whose crew had received the 1995-96 influenza vaccine. We describe the details of this outbreak.
The USS Arkansas is a nuclear-powered, guided missile cruiser with a complement of >500 men. It was in its home port of Bremerton, Washington, in January 1996. On February 1, this cruiser and her sister ship, the USS California (crew >550 men), departed for a 3-week training exercise in the waters off southern California. Up to departure date, the primary contact the two crews had was a shared dining facility. On February 5, the USS Arkansas contacted the Navy Environmental and Preventive Medicine Unit in San Diego, California (NEPMU-5), to report the onset of an acute febrile respiratory disease in many crew members. Subsequently, high rates of an incapacitating illness forced the ship to dock in San Diego, where it remained for 2 days.
On February 6, >60 crew members reported to the USS Arkansas medical department with respiratory symptoms; the 16 most symptomatic patients underwent a complete medical examination and additional laboratory testing. Pharyngeal swabs were collected from 50 ill crew members and submitted for viral culture. After influenza A was diagnosed, amantadine was flown to the ship and offered, 100 mg twice a day, to unvaccinated persons and those in the first days of illness. Follow-up surveys and interviews were conducted in early March after the ship returned from its training exercises. The crew's medical, berth, work site, and immunization records were reviewed.
Case Finding
Several case-finding methods were used in this outbreak. Initially, cases were identified by the ship's medical officer and corpsmen. Their case definition included crew members seeking medical evaluation with symptoms of fever, headache, sore throat, and/or cough along with nonspecific symptoms of fatigue and malaise. NEPMU-5, notified of the outbreak, sent an epidemiology team to meet the ship. This team used a symptom-based questionnaire, administered to the entire crew at the outbreak peak (February 6), to seek additional cases.
On February 23, the ship returned to Bremerton. A follow-up investigation then took place, which included review of sick-call logs and medical department reports, interviews with select crew members, and a follow-up questionnaire given to all crew members onboard during the outbreak. This questionnaire sought to identify the impact of the outbreak on the ship's function and the effects of intervention with amantadine. Those persons who reported a influenza-like illness on the follow-up questionnaire and had not yet completed the initial questionnaire were asked to do so.
A person was considered a case if he had a documented illness consistent with influenza or if he had indicated on the initial questionnaire that he had had an influenza-like illness and associated symptoms in the first 3 weeks of February. A possible case was a person who had an influenza-like illness but either did not complete the symptom questionnaire or completed it before becoming ill.
Fifty pharyngeal swabs collected in viral transport media were submitted for culture to the virology lab of Green Hospital at Scripps Clinic in La Jolla, California. Influenza A virus was identified by immunofluorescent staining of infected cell cultures with commercially available monoclonal antibodies. Successfully cultured viruses were forwarded to San Diego County's public health department laboratory for identification of influenza A subtype. The laboratory forwarded five of these isolates to CDC for antigenic characterization by hemagglutination-inhibition reactions with ferret antisera directed against a reference battery of influenza viral antigens, including currently circulating strains received from the global World Health Organization (WHO) influenza network.
A total of 548 Navy crew members and 3 civilians were onboard February 1-23; 440 crew members (80%) completed the first questionnaire and 509 (93%) completed the follow-up questionnaire. All respondents were male, age 18 to 48 years (mean 26 ± 6 years). Some 523 crew members (95%) had received the 1995-96 influenza vaccine in December 1995. Administration had been uniform, and the cold chain was maintained. The vaccine lot number and administration time were the same as those used for the crew of the companion ship, the USS California.
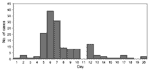
A total of 232 USS Arkansas crew members were identified with an influenza-like illness during the outbreak (attack rate = 42%); 158 cases were identified by the medical department; 74 patients did not seek medical attention but met case criteria. An additional 63 crew members (11%) reported having some influenza-like symptoms (probable cases) but did not meet the definite case criteria. The outbreak peaked on February 6; the last case was identified on February 20 (Figure 1). Review of medical logs and interviews with the medical department and crew of the USS California revealed that no one aboard that ship had sought medical attention for influenza-like illness during the training exercise.
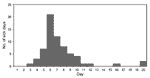
Ill members of the Arkansas crew missed 106 working days (Figure 2). One patient with severe chest pain as a result of infection was medically evacuated by helicopter. An additional 8 work days were lost by probable cases (N = 63).
After influenza A was identified in the crew, amantadine, 100 mg twice a day for 5 days, was offered to all unvaccinated persons (until the outbreak subsided) and to those in the first 2 days of illness. A total of 59 persons chose to take the antiviral, 49 for therapy and 10 for prophylaxis; 11 of 59 had side effects (insomnia, 4; headache, 4; nausea, 2; dizziness, 1; and bad dreams, 1). One person discontinued therapy because of headaches. A total of 28 unvaccinated crew members were onboard; 11 (61%) of the 18 unvaccinated persons who did not take amantadine met case criteria for influenza, but none of 10 unvaccinated persons who took amantadine prophylaxis had influenza-like symptoms (p-value <0.03, Fisher's exact test, 1-tailed).
Interviews and record reviews yielded a possible explanation for an outbreak's occurring on the Arkansas but not on the California, a ship with the same home port and vaccination schedule and a crew in close proximity until the day of departure. A sailor returned by plane to Bremerton from vacation in North Carolina on January 27 and became ill on January 28. He reported to the USS Arkansas on the day of departure (February 1). On January 28, a second sailor visited the first sailor in his home. The second sailor became ill on January 31 and was immediately placed on the sick list upon reporting to the Arkansas on February 1. On February 3, members of the crew who worked and lived in the same parts of the ship as the second sailor became ill. The outbreak peaked 3 days later. This correlated with a 3-day incubation period and efficient transmission. Four of six members of the investigating team (all Navy personnel vaccinated with the 1995-96 influenza vaccine) had an influenza-like illness that appeared to respond to amantadine therapy.
Influenza A was isolated from 30 of 50 throat culture specimens. They were confirmed as influenza A (H3N2) by the San Diego County Public Health Laboratory. All five isolates submitted to CDC were antigenically characterized as A/Wuhan/359/95-like.
This outbreak demonstrates the potential for rapid spread of influenza A throughout a confined population despite appropriate vaccination. The efficiency of human-to-human transmission is emphasized by the fact that there was no discernible difference in attack rates between various areas of the ship by the end of the outbreak. Although over 95% of the Arkansas crew were appropriately immunized with the 1995-96 influenza vaccine, at least 42% became ill with influenza; when definite and probable cases were included, the attack rate was 54%, for an estimated 46% efficacy of the 1995-96 influenza vaccine against the Wuhan strain.
The outbreak reiterates that optimal prevention of influenza by vaccination depends on the antigenic fit between the vaccine strain and the infecting virus, which, in turn, is dependent on the early identification of drift variants. The identification of the drift variant, A/Wuhan/359/95(H3N2), as the causative pathogen underscores the need for increasing the number as well as the capabilities of surveillance laboratories worldwide to rapidly isolate and identify strains of currently circulating influenza viruses. In recent years, the Department of Defense has augmented CDC and WHO surveillance efforts through establishing a collaborative network of >30 domestic and international influenza surveillance sites (8,9). Influenza isolates from this surveillance have proven valuable in decisions about vaccine content.
Dr. Earhart is an infectious disease attending physician at Naval Medical Center San Diego. His academic interests include emerging diseases with military relevance.
Acknowledgments
We thank the medical department of the USS Arkansas for their assistance with data collection; the crews of the USS Arkansas and USS California for their cooperation during the investigation; Dr. Helen Regnery and the Influenza Branch, Strain Surveillance Section, CDC, for antigenic characterization; and Capt. Patrick E. Olson, MC, U.S. Navy, for critical review of the manuscript.
This research was conducted in compliance with all applicable federal regulations governing the protection of human subjects in research. This represents report no. 00-20, supported by Department of Defense's Global Emerging Infections System and the Navy Bureau of Medicine and Surgery, Department of the Navy, Washington, DC, under work unit no. reimbursable-6609.
References
- White DC. A shipboard epidemic of Asian influenza. U S Armed Forces Med J. 1957;8:1717–25.PubMedGoogle Scholar
- Ninety-first annual report of the Surgeon General, US Navy: Statistics of diseases and injuries in the United States Navy, calendar year 1955. Washington: Department of the Navy, Bureau of Medicine and Surgery; 1956.
- Seal JR. Reactions to influenza vaccine. U S Armed Forces Med J. 1955;6:1559–63.PubMedGoogle Scholar
- Immunizations and chemoprophylaxis. Bureau of Medicine and Surgery Instruction 6230.15. Washington: Department of the Navy, Bureau of Medicine and Surgery;1995.
- Marwick C. Antigenically different subtype in new flu vaccine. JAMA. 1996;275:825. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update: influenza activity--worldwide, 1996. MMWR Morb Mortal Wkly Rep. 1996;45:816–9.PubMedGoogle Scholar
- Gambel J, Shlim D, Canas LC, Cox NJ, Regnery HL, Scott RM, Partnerships for detecting emerging infectious diseases: Nepal and global influenza surveillance. Emerg Infect Dis. 1998;4:128–30. DOIPubMedGoogle Scholar
- Gray GC, Callahan JD, Hawksworth AW, Fisher CA, Gaydos JC. Respiratory diseases among U.S. military personnel: countering emerging threats. Emerg Infect Dis. 1999;5:379–85. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 7, Number 3—June 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kenneth Earhart, Division of Infectious Diseases, Naval Medical Center San Diego, San Diego, CA 92134,USA: fax: 619-532-7478
Top