Volume 7, Number 6—December 2001
Research
Detection and Identification of Spotted Fever Group Rickettsiae and Ehrlichiae in African Ticks
Cite This Article
Citation for Media
Abstract
Rickettsia africae, a recently identified pathogen, was detected for the first time in Amblyomma ticks from Niger, Mali, Burundi, and Sudan, and "R. mongolotimonae" was identified for the first time in Africa. Rickettsiae of unknown pathogenicity and two new ehrlichiae of the Ehrlichia canis group were identified in ticks from Mali and Niger.
Spotted fever group rickettsiae and ehrlichiae are obligate intracellular gram-negative bacteria associated with arthropods, mainly ticks. While feeding, ticks can transmit these microorganisms to humans and animals (1). Two human tick-borne rickettsioses are known to occur in Africa (2). Mediterranean spotted fever, caused by Rickettsia conorii, is transmitted by the brown dog tick, Rhipicephalus sanguineus, which is well adapted to urban environments. R. conorii is prevalent in the Mediterranean area (Tunisia, Algeria, Morocco, Libya, and Egypt) and has also been isolated or detected in Kenya, Central Africa, Zimbabwe, and South Africa (2). Although African tick bite fever has been recognized since the beginning of the century as a rural disease usually contracted from ticks of cattle and game, it was regarded as synonymous with Mediterranean spotted fever, until the first human infection with R. africae was reported from Zimbabwe in 1992. Subsequently, numerous cases have been reported in tourists returning from southern Africa, where the cattle tick Amblyomma hebraeum is the vector (2,3). R. africae has also been recovered from A. variegatum ticks in Ethiopia and central Africa (2). In 1992, a survey for antibodies against Ehrlichia chaffeensis (the agent of human monocytic ehrlichiosis) in human sera from eight African countries indicated that human ehrlichioses might occur on the continent (4), and subsequently a case (diagnosed by serology only) was reported from Mali (5). Recently, new molecular methods have enabled the development of useful, sensitive, and rapid tools to detect and identify tick-borne pathogens in arthropods, including ticks (6). In this work, we tested ticks from Africa for rickettsial and ehrlichial DNA using polymerase chain reaction (PCR) and sequence analysis of amplified products.
Ticks were kept frozen at -20°C (in Niger) or at -80°C (in other countries) before being tested. DNA of each tick was extracted as described (7). Rickettsial and ehrlichial DNA was detected by PCR as described, using specific primers (Table).
The sequences of PCR products were obtained and analyzed with the corresponding sequences of rickettsial or ehrlichial species as described (7). Multiple alignment analysis was performed by using the ClustalW program version 1.8 in the DNA Data Bank of Japan (DDBJ; Mishima, Japan [http://www.ddbj.nig.ac.jp/htmls/E-mail/clustalw-e.html]). All sequences used in the study are available in GenBank; the accession numbers of the new genotypes detected in this work are shown in the Table footnotes.
Rickettsial DNA was detected in 24 (7.2%) of the 332 ticks examined (Table). R. africae was detected from A. variegatum from Mali (2/6), Niger (2/6), and Burundi (1/13), and from 1 of 16 A. lepidum from the Sudan. R. aeschlimmanii was detected in Hyalomma marginatum rufipes from Niger and Mali (8/24 and 3/20, respectively) and R. massiliae in 2/37 Rh. muhsamae from Mali. Further, three new ompA sequences (590 bp) were obtained from A. variegatum from Mali and Niger (Table). These were 99.3%-99.5% identical to those of R. africae. In the phylogenetic tree based on these ompA sequences, the three rickettsiae (named RAv1, RAv3, RAv9) were closely related to one another (95.7% bootstrap value) and branched with R. africae (86.1% bootstrap value) (data not shown). Partial sequences (316 bp) of the gltA gene of RAv1, RAv3, and RAv9 were also found to be closely related to those of R. africae (99% of similarity). Two new 16S rRNA ehrlichial genotypes were detected, including ERm58 (1,380 bp) in 7/37 Rh. muhsamae from Mali and EHt224 (1366 bp) in 1/5 H. truncatum from Niger. Both sequences were very similar (99.34% similarity), but different from those described for all the known ehrlichiae (i.e., 98.55% similarity with E. chaffeensis, 98.26% with E. canis and E. ewingii, and 97.75% with E. muris and Cowdria ruminantium). In a phylogenetic tree based on 16S rRNA gene sequences, ERm58 and EHt224 were found to be closely related and to belong to the E. canis group (data not shown). Enlarged gltA sequences of ERm58 (1,140 bp) and EHt224 (1,189 bp) were also obtained from the above ticks. Phylogenetic analyses of these sequences confirmed that ERm58 and EHt224 belonged to the E. canis group (data not shown).
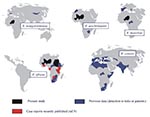
This study has shown for the first time that R. africae, the agent of African tick bite fever, is present in West Africa (Mali and Niger), the Sudan, and Burundi. It also indicates a potential role for A. variegatum and A. lepidum as vectors of R. africae in these areas. Recently, we also documented cases in tourists returning from numerous countries, including those in West and East Africa (9) (Figure). Our results support the hypothesis that the geographic distribution of African tick bite fever parallels that of the distribution of Amblyomma spp., as ticks are known to be vectors and also reservoirs of tick-borne rickettsiae (2). In Africa, although the principal vector of R. africae appeared to be A. hebraeum, which is prevalent in southern Africa, A. variegatum (which is widely distributed throughout sub-Saharan Africa) appears as a potential vector. Amblyomma are known to readily feed on people in Africa and are commonly infected with rickettsiae (up to 100%). Thus, African tick bite fever may have a high prevalence throughout the continent. Studies have shown seroprevalences of 30%-80% for spotted fever group rickettsiae in sub-Saharan Africa (2), although it is unclear what proportion of those infections might be due to R. africae infection.
In this study, we report for the first time the presence of "R. mongolotimonae" in Africa (Niger). This pathogen was first isolated from an H. asiaticum collected in Inner Mongolia, China. Later, the same agent was isolated from the blood and skin of a febrile woman from Marseille in 1996 (2), which demonstrated its pathogenicity for humans. Subsequently, we have recognized four more cases in southern France (10, and unpub. data). Results of this study suggest that R. mongolotimonae may be associated with Hyalomma sp. ticks throughout the world. In this work, we also detected two rickettsiae of unknown pathogenicity, namely R. aeschlimannii and R. massiliae. Although this is the first recognition of these rickettsiae in Mali and Niger, the epidemiologic importance of this finding has yet to be determined. Finally, we detected three new spotted fever group genotypes closely related to R. africae. Until further studies clarify the position of these organisms, we suggest they may be considered variant strains of R. africae.
Although previous reports, based on the results of serosurveys, have indicated that human ehrlichioses occur in Africa, firm evidence is still absent. Because of the serologic cross-reactivity between ehrlichiae, serosurvey results have to be interpreted carefully. We detected two new ehrlichial genotypes, that is, Erm58 in Rh. muhsamae from Mali and Eht224 in H. truncatum from Niger. Both belong to the E. canis group, which includes E. chaffeensis, C. ruminantium, E. muris, E. ewingii, and a new isolate detected in Japanese ticks (11). In this group, as within each group of ehrlichiae, members share homologous surface antigens and thus cross-react extensively in serologic assays (12). Erm58 and Eht224 may also be organisms responsible for such serologic cross-reactions, including in serosurveys and case reports of human ehrlichioses in Africa. In 1997, new ehrlichial genotypes were also detected in Namibia and Zimbabwe, and a number of ehrlichiae in Africa may be responsible for serologic cross-reactions in serosurveys of humans and animals for currently recognized pathogenic ehrlichiae (13,14). The pathogenicity of the Erm58 and Eht224 recognized in our study has yet to be determined, and further studies to characterize the human ehrlichioses in Africa are indicated. Moreover, it remains to be demonstrated whether H. truncatum and Rh. muhsamae ticks act as vectors or reservoirs of the new ehrlichiae, since ticks also could have been infected while feeding on bacteremic mammals.
Although this study detected for the first time certain richettsiae and ehrlichiae in African countries, systematic sampling was not done, and results cannot address their prevalence and distribution. However, this work provides a starting point for epidemiologic studies there.
Dr. Philippe Parola obtained both MD and PhD degrees at the Faculty of Medecine of Marseille, France, and is now a postdoctoral fellow at the Harvard School of Public Health, Boston, Massachusetts. His research interests include medical entomology and tick-borne diseases.
Acknowledgments
We thank Djibo Garba, Issa Baradji, Salif Diagana, Jean-Bosco Ndihokubwayo, Bernard Davoust, Michael Cino, and Patricia Amargier for their help in collecting the ticks. We are grateful to Patrick Kelly for reviewing the manuscript.
This study was funded partly by French Ministry of Research and Technology ("Programme de recherche fondamentale en microbiologie et maladies infectieuses et parasitaires 2000"). During part of the field work, Dr. Parola was supported by Université de la Méditerranée.
References
- Parola P, Raoult D. Ticks and tick-borne bacterial human diseases, an emerging infectious threat. [published erratum appears in Clin Infect Dis 2001;33:749]. Clin Infect Dis. 2001;32:897–8. DOIPubMedGoogle Scholar
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719.PubMedGoogle Scholar
- Fournier PE, Beytout J, Raoult D. Related tick-transmitted infections in Transvaal: consider Rickettsia africae. Emerg Infect Dis. 1999;5:178–81. DOIPubMedGoogle Scholar
- Brouqui P, Le Cam C, Kelly PJ, Laurens R, Tounkara A, Sawadogo S, Serologic evidence for human ehrlichiosis in Africa. Eur J Epidemiol. 1994;10:695–8. DOIPubMedGoogle Scholar
- Uhaa IJ, MacLean JD, Greene CR, Fishbein DB. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg. 1992;46:161–4.PubMedGoogle Scholar
- Sparagano OA, Allsopp MT, Mank RA, Rijpkema SG, Figueroa JV, Jongejan F. Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): a review. Exp Appl Acarol. 1999;23:929–60. DOIPubMedGoogle Scholar
- Rydkina E, Roux V, Fetisova N, Rudakov N, Gafarova M, Tarasevich I, New Rickettsiae in ticks collected in territories of the former Soviet Union. Emerg Infect Dis. 1999;5:811–4. DOIPubMedGoogle Scholar
- Parola P, Roux V, Camicas JL, Brouqui P, Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans R Soc Trop Med Hyg. 2000;94:707–8. DOIPubMedGoogle Scholar
- Raoult D, Fournier PE, Fenollar F, Jensenius M, Prioe T, de Pina JJ, Rickettsia africae a tick-borne pathogen in travelers to sub-Saharan Africa. N Engl J Med. 2001;344:1504–10. DOIPubMedGoogle Scholar
- Fournier PE, Tissot-Dupont H, Gallais H, Raoult D. Rickettsia mongolotimonae: a rare pathogen in France.. Emerg Infect Dis. 2000;6:290–2. DOIPubMedGoogle Scholar
- Inokuma H, Parola P, Raoult D, Brouqui P. Molecular survey of Ehrlichia infection in ticks from animals in Yamagushi prefecture, Japan. Vet Parasitol. 2001;99:335–9. DOIPubMedGoogle Scholar
- Rikihisa Y. Ehrlichiae of veterinary importance. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millennium. Paris: Elsevier; 1999. p. 393-405.
- Allsopp M, Visser ES, du Plessis JL, Vogel SW, Allsopp BA. Different organisms associated with heartwater as shown by analysis of 16S ribosomal RNA gene sequences. Vet Parasitol. 1997;71:283–300. DOIPubMedGoogle Scholar
- Savadye DT, Kelly PJ, Mahan SM. Evidence to show that an agent that cross-reacts serologically with Cowdria ruminantium in Zimbabwe is transmitted by ticks. Exp Appl Acarol. 1998;22:111–22. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 7, Number 6—December 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Didier Raoult, Unité des Rickettsies, Faculté de Médecine, Université de la Méditerranée, CNRS UMR 6020, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France; fax: 33-491-83-0390
Top