Volume 31, Number 9—September 2025
Research Letter
Human Babesiosis Caused by Babesia venatorum, Russia, 2024
Cite This Article
Citation for Media
Abstract
We report a case of acute babesiosis in a splenectomized 63-year-old man in Siberia, Russia. We confirmed the causative agent, Babesia venatorum, by PCR. Our study demonstrated a change in the structure of the parasite population, from single parasite invasion of erythrocytes to multioccupancy, without an increase in parasitemia level.
Babesiosis is an emerging tickborne infection caused by intraerythrocytic protozoa. To date, researchers have described more than 50 cases of babesiosis in humans in Europe, almost always fulminant in splenectomized patients and typically attributed to Babesia divergens. Some recent reports also describe several cases of human infection with B. venatorum, associated with milder infections than those caused by B. divergens (1). Researchers have also described sporadic cases of babesiosis caused by infection with Babesia microti and B. divergens in Asia-Pacific regions (2,3), but the practically asymptomatic course of the human infection with B. venatorum is more common (4). Although reports have noted detection of Babesia spp. DNA in Ixodes persulcatus ticks in Siberia (5), cases of human infections have yet to be reported in that region of Russia.
We report the case of a 63-year-old man who resided in a forested, mountainous area of Khakassia, East Siberia, Russia, and had undergone splenectomy. On September 30, 2024, the man sought treatment for an influenza-like syndrome with signs and symptoms that included a fever of 38°C, severe general weakness, darkening of urine, a decrease in diuresis, jaundice, dyspnea, and stomachache. Attending physicians admitted the patient to the hospital on October 2, 2024 (Table). The patient reported no awareness of a tick bite and had received no blood products in the previous 3 months.
Blood smears obtained on hospital admission tested positive for Plasmodium spp. However, because the patient reported no recent travel to malaria-endemic areas, we sent the blood samples for retesting at Sechenov University (Moscow, Russia), where results confirmed babesiosis. We then examined the blood smears after Romanovsky-Giemsa staining, noting the paired forms diverging at a wide angle (≤180°), which is characteristic of both B. divergens and B. venatorum (Appendix Figure 1).
We screened DNA samples extracted from the blood smears for B. divergens, B. microti, and B. venatorum by PCR, using methods described previously (6). We partially sequenced the 18S rRNA gene (1,112 bp; GenBank accession no. PV086113) (5). We aligned, compared, and analyzed the resulting nucleotide sequences and reference sequences downloaded from GenBank by using MEGA X (7). We also reconstructed a phylogenetic tree (Appendix Figure 2). Using forward and reverse primers form 18S RNA of Babesia spp. from Europe, we were able to detect only B. venatorum DNA.
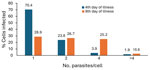
We started etiotropic treatment for the patient 3 days from the time we initially detected intraerythrocytic parasites and identified the causative agent as Babesia spp. The patient responded to therapy (clindamycin and quinine sulfate), and by day 9 of treatment, parasites were no longer detectable with microscopy (Table). We noted that the ratio of erythrocytes invaded by >1 trophozoite changed as the infection progressed. Four days after symptom onset, single trophozoites and pairs (figure 8 pattern) predominated. On the 8th day of infection, with the same level of parasitemia, almost half of the infected erythrocytes contained ≥4 trophozoites (Figure). Parasites continued to divide intensively but did not leave the host cell. Prior research has noted multiple parasites present in individual erythrocytes during fulminant infections in humans (8) and in heavily infected in vitro cultures (9). Results of such studies suggests that multioccupancy of trophozoites in the erythrocyte prevents a sharp increase in parasitemia and helps to preserve the parasite population in the host (9). However, the phenomenon we observed occurred at both high (>23%) and low (<3%) parasitemia.
Our study of this unique case of human babesiosis in Siberia, Russia, provides molecular evidence that the etiologic agent was B. venatorum. Prior research noted the Asia variant of B. venatorum in asymptomatic persons from China (4). B. divergens infection in splenectomized humans could lead to death, even with timely treatment (1). However, the similar course and positive outcome of babesiosis in our patient resembled those in cases previously reported in Europe (1), suggesting that the causative agent of the disease was B. venatorum, which we confirmed by PCR.
Our patient resided in a village located in a forested area, which is a natural habitat for ticks. The man’s work involved staying in the forest, again increasing his risk for tick bites. The fact that the patient did not notice a tick bite is not unusual. Only 50% to 70% of patients with tickborne diseases recall being bitten by a tick (2). Considering the ability of B. venatorum to be transmitted transovarially and transstadially within I. persulcatus ticks, it is possible for a person to become infected through the bite of a tick nymph, which is smaller and less noticeable than the adult (10).
In conclusion, our case study revealed the potential risk of B. venatorum infection for persons living in Siberia, Russia. Clinicians should be aware that infection can occur as an influenza-like illness and may go unnoticed in immunocompetent persons.
Dr. Zelya is a parasitologist working as an assistant professor at Martsinovsky Institute of Medical Parasitology, Tropical and Vector-Borne Diseases, Sechenov University, Moscow, Russia. Her primary research interests are centered on laboratory diagnostics and epidemiology of parasitic diseases.
Acknowledgment
We are deeply grateful to Svetlana L. Lukovenko for her collaboration in preparing and sending us blood products (thick and thin blood films) with intraerythrocytic protozoa.
References
- Hildebrandt A, Zintl A, Montero E, Hunfeld K-P, Gray J. Human babesiosis in Europe. Pathogens. 2021;10:1165. DOIPubMedGoogle Scholar
- Zhou X, Xia S, Huang JL, Tambo E, Zhuge HX, Zhou XN. Human babesiosis, an emerging tick-borne disease in the People’s Republic of China. Parasit Vectors. 2014;7:509. DOIPubMedGoogle Scholar
- Kumar A, O’Bryan J, Krause PJ. The global emergence of human babesiosis. Pathogens. 2021;10:1447. DOIPubMedGoogle Scholar
- Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis. 2015;15:196–203. DOIPubMedGoogle Scholar
- Rar VA, Epikhina TI, Livanova NN, Panov VV. Genetic diversity of Babesia in Ixodes persulcatus and small mammals from North Ural and West Siberia, Russia. Parasitology. 2011;138:175–82. DOIPubMedGoogle Scholar
- Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, et al. High-throughput screening of tick-borne pathogens in Europe. Front Cell Infect Microbiol. 2014;4:103. DOIPubMedGoogle Scholar
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9. DOIPubMedGoogle Scholar
- Kukina IV, Zelya OP. Extraordinary high level of propagation of Babesia divergens in severe human babesiosis. Parasitology. 2022;149:1160–3. DOIPubMedGoogle Scholar
- Lobo CA, Cursino-Santos JR, Singh M, Rodriguez M. Babesia divergens: a drive to survive. Pathogens. 2019;8:95. DOIPubMedGoogle Scholar
- Bonnet S, Brisseau N, Hermouet A, Jouglin M, Chauvin A. Experimental in vitro transmission of Babesia sp. (EU1) by Ixodes ricinus. Vet Res. 2009;40:21. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: August 19, 2025
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Olga P. Zelya, Martsinovsky Institute of Medical Parasitology, Tropical and Vector-Borne Diseases, Sechenov University, Malaya Pirogovskaya st., 20-1, Moscow 119435, Russia
Top