Volume 8, Number 5—May 2002
Research
Genetic Variants of Ehrlichia phagocytophila1, Rhode Island and Connecticut
Cite This Article
Citation for Media
Abstract
Primers were used to amplify a 561-bp region of the 16S rRNA gene of Ehrlichia phagocytophila from Ixodes scapularis ticks and small mammals collected in Rhode Island and Connecticut. DNA sequences for all 50 E. phagocytophila-positive samples collected from 1996 through 1998 in southwestern Connecticut were identical to the sequence reported for E. phagocytophila DNA from confirmed human cases. In contrast, the sequences from 92 of 123 E. phagocytophila-positive Rhode Island samples collected from 1996 through 1999 included several variants differing by 1-2 nucleotides from that in the agent infecting humans. While 11.9% of 67 E. phagocytophila-positive ticks collected during 1997 in Rhode Island harbored ehrlichiae with sequences identical to that of the human agent, 79.1% had a variant sequence not previously described. The low incidence of human ehrlichiosis in Rhode Island may in part result from interference by these variant ehrlichiae with maintenance and transmission of the true agent of human disease.
Members of the genus Ehrlichia are obligate, intracellular bacteria in the order Rickettsiales. Although ehrlichial infections of veterinary importance were first described in 1935, the first case of human ehrlichiosis in the United States was reported in 1987 (1). The human pathogen was subsequently identified as Ehrlichia chaffeensis (2), and the number of reported human cases now exceeds 740 (3). In 1994, a second ehrlichial infection in humans was reported, called human granulocytic ehrlichiosis (HGE) because of its proclivity to infect neutrophils (4). Most HGE cases have been diagnosed in the northeastern and upper midwestern United States, although a few cases have been reported in Europe and northern California (5–12).
The close genetic and antigenic relationship of the HGE agent to two previously characterized species (E. phagocytophila, noted for infections of ruminants in Europe, and E. equi, the agent of equine granulocytic ehrlichiosis) has led to the suggestion that these three be classified as a single species, with E. phagocytophila as the precedent name. The 16S rRNA gene has been amplified and sequenced from confirmed human cases in both North America and Europe, and all sequences have been identical to the original published sequence for the HGE agent (4,8), with the exception of two cases recently reported from northern California that were the same as the E. equi 16S rRNA gene sequence (12). A variant that differed by 2 bp from the sequence of the HGE agent was reported in white-tailed deer in Maryland and Wisconsin and in Ixodes scapularis ticks collected in Rhode Island (13,14). Likewise, the 16S rRNA sequences determined from documented infections of horses and ruminants by various E. phagocytophila strains have differed by several bp from the sequences of the HGE agent. None of the variant forms have been shown to cause human disease. Another ehrlichia found in nature, which is closely related to E. phagocytophila but apparently does not cause human disease, is the white-tailed deer agent (15). Ehrlichiae closely related to E. phagocytophila recently have been identified in Colorado, where human ehrlichiosis is not endemic (16). These data suggest that only a subset of the E. phagocytophila strains that exist in nature can cause human disease. Although the HGE agent is now considered a member of the species E. phagocytophila, hereafter we will designate the isolates responsible for human disease as E. phagocytophila-human agent (EP-ha) to differentiate them from the 16S rRNA sequence variants we describe in this report.
Rhode Island and Connecticut, adjacent northeastern states, have similar frequency and distribution of vector I. scapularis ticks and primary reservoir hosts (white-footed mice and chipmunks) (17–19). However, the number of reported human infections with E. phagocytophila is dramatically higher in Connecticut than in Rhode Island. Through 1997, the average annual incidences (per one million population) of HGE in Connecticut and Rhode Island were 15.90 and 0.67, respectively (3). We investigated the frequency and distribution of E. phagocytophila, including EP-ha and E. phagocytophila-related variants, in potential reservoir and vector populations in Rhode Island and Connecticut.
Tick and Mammal Collections
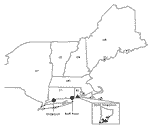
Questing nymphal and adult black-legged ticks (I. scapularis) collected from four sites in South Kingston, Rhode Island, and one site in Bridgeport, Connecticut, were analyzed for the presence of granulocytic ehrlichiae (Figure). Collections of adult- or nymphal-stage ticks or both were available from ongoing tick surveillance conducted in each region from 1996 to 1999. Questing nymphal ticks collected from Bluff Point in southeastern Connecticut in 1997 were also available. The Rhode Island sites are all located in the state’s zone of highest I. scapularis density (17). Tick density was also high at the Bridgeport site. Ticks were collected by following standardized sampling procedures (17). All ticks were stored for <2 years in 70% ethanol until tested. Small rodents, including white-footed mice (Peromyscus leucopus) and chipmunks (Tamias striatus) live-trapped at the same locations were bled following procedures approved by the institutional animal care and use committees of each institution. Briefly, animals were trapped from July to September along transect lines or in trapping grids. An additional collection was made during May 1998 at the Bridgeport site. Blood was stored in EDTA at -80°C until tested for ehrlichiae by polymerase chain reaction (PCR) techniques and DNA sequencing.
Sample Preparation
DNA was extracted directly from blood samples by using a QIAamp Blood Extraction Kit (Qiagen, Chatsworth, CA), according to manufacturer’s instructions. Briefly, detergent lysis was done in the presence of proteinase K for 10 min at 70°C. The lysed material was applied to a spin column containing a silica gel-based membrane and then washed twice. Purified DNA was eluted from the columns in 200 µL Tris-HCl (10 mM, pH 8.0) and stored at 4°C until used as template for PCR amplification. DNA was extracted from I. scapularis ticks by a modification of the manufacturer’s protocol for the QIAamp Tissue Kit (Qiagen) as described (13).
PCR Analysis
A nested PCR that amplified a 546-bp portion from the 5´ region of the 16S rRNA gene was used to identify granulocytic ehrlichiae in tick and wildlife samples (13). Briefly, primary amplifications consisted of 40 cycles in a Perkin-Elmer 9600 thermal cycler (Perkin-Elmer, Applied Biosystems Division, Foster City, CA), with each cycle consisting of a 30-sec denaturation at 94°C, 30-sec annealing at 55°C, and a 1-min extension at 72°C. The 40 cycles were preceded by a 2-minute denaturation at 95°C and followed by a 5-minute extension at 72°C. Primary amplifications used primers ge3a and ge10r and reagents from the GeneAmp PCR Kit with AmpliTaq DNA Polymerase (Perkin-Elmer). Each reaction contained 5 µL of purified DNA as template in a total volume of 50 µL, as well as 200 µM each deoxynucleoside triphosphate (dNTP) (dATP, dCTP, dGTP, and dTTP), 1.25 units Taq polymerase, and 0.5 µM each of primer. Reaction products were subsequently maintained at 4°C until analyzed by agarose gel electrophoresis or used as template for nested reactions.
Nested amplifications used primers ge9f and ge2 and 1 µL of the primary PCR product as template in a total volume of 50 µl. Each nested amplification contained 200 µM each dNTP (dATP, dCTP, dGTP, and dTTP), 1.25 units Taq polymerase, and 0.2 µM each of primer. Nested cycling conditions were as described for the primary amplification, except 30 cycles were used. Reactions were subsequently maintained at 4°C until analyzed by agarose gel electrophoresis or purified for DNA sequencing (13).
DNA Sequencing and Data Analysis
All samples producing positive PCR products were subjected to DNA sequencing reactions with fluorescent-labeled dideoxynucleotide technology (Dye Terminator Cycle Sequencing Ready Reaction Kit, Perkin-Elmer, Applied Biosystems Division). Sequencing reaction products were separated, and data were collected with an ABI 377 automated DNA sequencer (Perkin-Elmer, Applied Biosystems Division). The full sequence was determined for both strands of each DNA template to ensure maximum accuracy of the data. Sequences were edited and assembled with the Staden software programs (20) and analyzed with the Wisconsin Sequence Analysis Package (Genetics Computer Group, Madison, WI) (21).
Fifty (13.3%) of 375 I. scapularis ticks from Bridgeport, Conn., were PCR positive for ehrlichiae (Table 1). The percentage positive in each of the 4 years ranged from 6.1% in nymphs in 1998 to 23.3% in adults in 1996. Less year-to-year variation was noted in adult ticks, in which infection prevalence ranged from 11.7% (1997) to 23.3% (1996). PCR analysis of EDTA blood samples from white-footed mice collected in Connecticut during summer and fall 1997 and spring 1998 showed that 17 (36.2%) of 47 in 1997 and 3 (60%) of 5 in 1998 were positive (22). The amplification products were sequenced for each Ehrlichia PCR-positive mouse and tick. All products from samples collected in the Bridgeport, Conn., area from 1996 through 1998 had sequences identical to the 16S rRNA gene (EP-ha) previously amplified and sequenced from documented human infections in the Northeast and Upper Midwest United States and in Europe (4). The 16S rRNA sequence determined from adult ticks collected from Bridgeport in 1999 showed that all 12 positive samples also contained the human agent (EP-ha), although one of the ticks produced a mixed sequence, suggesting the presence of more than one agent. The PCR products from this tick were cloned, and individual clones were purified and sequenced. These data confirmed the presence of a mixed population of ehrlichiae containing some 16S rRNA sequences that matched EP-ha and some that differed from EP-ha by two nucleotides. The latter sequence was identical to a variant (called variant 1) previously described in ticks in Rhode Island and deer in Maryland and Wisconsin (13,14) (Table 2). In contrast to ticks and rodents from the Bridgeport area, nymphal ticks collected in 1997 from Bluff Point in southeastern Connecticut contained a nearly equal distribution of EP-ha (5 [55.6%] of 9 positives) and variant 1 (4 [44.4%] of 9 positives) ehrlichiae.
Rhode Island samples from I. scapularis ticks, white-footed mice, and chipmunks contained E. phagocytophila variants as well as EP-ha. A total of 123 (22.9%) of 538 ticks were positive for E. phagocytophila by PCR, including 61 (26.3%) of 232 adults and 62 (20.3%) of 306 nymphs. DNA sequencing was performed on 92 of these PCR products, and overall, only 24 (26.1%) showed sequences identical to those of EP-ha. Fifteen (16.3%) ticks showed sequences corresponding to variant 1. The rest of the ticks (53 [57.6%]) had a novel sequence differing from EP-ha by 2 nucleotides and from variant 1 by 4 nucleotides (hereafter called variant 2) (Table 2).
PCR testing of blood samples from 19 Rhode Island chipmunks in 1996 detected 11 (57.9%) positives. DNA sequencing of these PCR products showed that nine were identical to the sequence of EP-ha; the remaining two represented novel variant sequences, each differing from EP-ha by a single nucleotide (variants 3 and 4; Table 2). Although both the white-tailed deer agent and the E. equi/CA human sequence variant (Table 2) are amplified by the PCR assay used in this study, neither agent has been detected in potential rodent reservoir populations in Connecticut or Rhode Island. Host and vector associations of EP-ha and the four E. phagocytophila variants found in Rhode Island are shown in Table 3.
The prevalence of E. phagocytophila in I. scapularis ticks (adults and nymphs combined, years 1996-1999) was higher in Rhode Island (22.8%) than in Bridgeport (13.3%) (p<0.001; Fisher’s exact test). This finding was also true for adult ticks: 26.3% were infected in Rhode Island compared with 15.4% in Bridgeport (p=0.002). Using either the total number of ticks tested or adult ticks only, the prevalence of E. phagocytophila in Rhode Island compared with Bridgeport was 1.7. However, if the 1997 Rhode Island data, which were skewed by an unusually large number of variants, are removed from the calculations, the percentage of E. phagocytophila-positive ticks (adults and nymphs) was significantly higher in Bridgeport (13.3%) than in Rhode Island (8.2%) (p=0.03). The same analysis, when restricted to the adult tick population, showed no significant difference between Connecticut (15.4%) and Rhode Island (13.4%) EP-positive ticks (p=0.6). If the 1997 Rhode Island data are excluded, the prevalence of E. phagocytophila in that state compared with Bridgeport was 0.6 for the total number of ticks tested and 0.8 for adult ticks only.
Infection prevalence data were available for adult and nymphal ticks from the same site for 3 years: Rhode Island in 1997 and 1999 and Bridgeport in 1998 (Table 1). For two of these, Rhode Island in 1999 and Bridgeport in 1998, the prevalence of E. phagocytophila was significantly higher (Rhode Island 1999; p=0.01) or borderline higher (Connecticut 1998; p=0.065) in adults than in nymphs. E. phagocytophila infection rates in nymphal and adult ticks from Rhode Island in 1997 did not differ significantly (p=0.21).
Temporal trends in tick infection rates showed that the prevalence of E. phagocytophila in Rhode Island nymphs was highest in 1997 and then declined in 1998 and 1999 (p<0.001; chi-square test for trend). However, the high number of variants found in both adult and nymphal ticks from Rhode Island in 1997 influenced this analysis, as E. phagocytophila prevalence in Rhode Island in 1997 was significantly higher than in all other years combined (p<0.001). In Bridgeport, no significant temporal trends were noted in E. phagocytophila infection rates in adult ticks (p=0.3), nor were significant prevalence or temporal trends noted in the rodents tested from any of the sites.
Analysis of the proportion of E. phagocytophila-positives that were variants showed that prevalence of the variants in Rhode Island (73.9% variants) was significantly higher than in Bridgeport (0.02% variants) (p<0.001). When the proportion of E. phagocytophila-positives that were variants was compared with the total number of positives for the two Connecticut sites, the ticks from Bluff Point (44.4% variants) showed significantly higher rates than ticks from Bridgeport (0.02% variants) (p<0.001).
Strains of E. phagocytophila found in nature are capable of causing disease in sheep, cattle, horses, dogs, cats, and humans. These strains, including the species previously known as the HGE agent and E. equi, are grouped as a single species on the basis of their close relationship at the genetic and antigenic levels. However, biological and ecological differences clearly exist between strains of E. phagocytophila, including varying host pathogenicity, vectors, DNA sequence, and geographic distribution. Small ribosomal subunit (16S) DNA sequences are highly conserved in bacteria and are often used to identify and differentiate bacterial species. The 16S rRNA gene sequences amplified from every confirmed human case, except for two isolated cases in northern California, have been identical to the E. phagocytophila-human agent (EP-ha) sequence determined by Chen et al. (4). PCR-positive white-footed mice (n=20) and I. scapularis ticks (n=38) collected in Bridgeport from 1996 through 1998 also harbored only E. phagocytophila identical in sequence to EP-ha for a 546-bp region of the 16S rRNA gene (4). Sequence analysis of PCR products from two Connecticut deer blood samples showed DNA identical to the EP-ha p44 gene sequence (23). However, an Ehrlichia organism with a 16S rRNA gene sequence differing from EP-ha by a single nucleotide has been identified in white-tailed deer from Maryland and Wisconsin and in I. scapularis from Rhode Island (13,14).
In contrast to our results from Bridgeport, where we consistently found EP-ha, mice and ticks from Rhode Island had a significantly lower percentage of isolates identical to EP-ha, but several E. phagocytophila variants with novel 16S rRNA gene sequences. These data indicate that variant forms of E. phagocytophila, not yet associated with human or veterinary disease, frequently occur in Rhode Island. The same or additional E. phagocytophila variants may also occur in other regions of the United States, but this concept remains to be investigated.
Most PCR assays amplify products from the variant agents that are the same size as the EP-ha PCR product, so that variants are indistinguishable when the products are analyzed only by agarose gel electrophoresis. Therefore, results from other human-infection prevalence surveillance studies in ticks and rodents that have not included PCR product sequencing may be misleading. For example, if we had not sequenced our PCR products for 1997, we would have concluded that 46.4% of nymphal and 38.3% of adult I. scapularis ticks collected in southern Rhode Island were positive for EP-ha. Actually, only 11.9% of the positives that were sequenced and an estimated 5.0% of the total ticks tested were EP-ha positive, with the rest of the 1997 Rhode Island positives consisting of genetic variants not yet associated with human disease.
The 16S rRNA sequences obtained from tick and rodent samples collected from Bridgeport from 1996 through 1998 were identical to EP-ha. However, in 1999, one tick collected in that site was positive for both EP-ha and a variant (variant 1) previously found in Rhode Island. In a retrospective analysis of ticks collected at another eastern Connecticut site (Bluff Point) close to the Rhode Island border, variant 1 was found in 1997. Our inability to detect variant 1 despite extensive testing of samples collected in Bridgeport from 1996 to 1998 and its subsequent appearance in 1999 suggests that its geographic range may be expanding westward. Additional studies with larger sample sizes of ticks and rodents from Bridgeport and other locations in Connecticut are needed to assess the prevalence of EP-ha and the variants, as our Bridgeport results may not be representative of the entire state. In fact, the Bluff Point data suggest that other areas of Connecticut may have EP-ha/variant populations quite different from those in Bridgeport and more similar to the distribution noted in Rhode Island.
The identification of the coinfected tick in Connecticut represents the first detection of more than one strain of E. phagocytophila in a single tick vector in the United States, although the coinfection of Ixodes ricinus ticks by 2 E. phagocytophila strains has been reported in Europe (24). These data indicate that two strains of the agent are capable of coexisting in a single tick, at least transiently, and that they can survive the molting process, since the coinfection was found in an unfed, host-seeking adult tick.
The results from Rhode Island samples collected in 1997 are unusual in several regards. First, the rate of E. phagocytophila-positive ticks (42.2% nymphs and adults) was very high relative to all other tick populations sampled from 1996 through 1999, and many of the positives were variant 2 (79.1% of PCR-positives sequenced). Second, the 1997 Rhode Island ticks represent the only population in which E. phagocytophila prevalence was higher in nymphs than adults (46.4% nymphs and 38.3% adults). Finally, variant 2 sequences were also seen in samples collected in 1997 from white-footed mice and chipmunks but were not detected before and have not been detected after 1997. The fact that both nymphal and adult questing ticks were positive for variant 2 suggests that the variant was present in reservoir species during both larval and nymphal feedings and may have been present in reservoirs from late summer 1996 through summer 1997. Why this variant appeared only in Rhode Island during 1997, was the most prevalent strain infecting ticks that year, and then completely disappeared are matters of speculation. Variant 2 may be a more common infection in a reservoir species that we did not examine, which may be less commonly targeted by host-seeking ticks. Expression of variant 2 in the tick population could have resulted if, during 1996-1997, host populations preferred by immature I. scapularis (i.e., white-footed mice and chipmunks) were lower than normal, resulting in a higher proportion of ticks feeding on such atypical hosts harboring variant 2. After molting, nymphs infected as larvae the previous year could have transmitted variant 2 to the more preferred hosts of immature ticks, resulting in the variant 2-positive mice found in 1997. Subsequent reestablishment of normal host populations may then have diluted the prevalence of variant 2, as immature tick feeding reverted to the preferred hosts.
Although the function and biological importance of the genetic differences in E. phagocytophila strains are unknown, we hypothesize that the variants may be interfering with maintenance and transmission of the human disease-causing agent (EP-ha). Even if an increased human ehrlichiosis case surveillance effort in Connecticut is taken into account, the number of confirmed and suspected cases differs dramatically between the two neighboring states: several hundred cases were reported in Connecticut compared with fewer than 25 cases in Rhode Island during the same time period. From 1995 to 1997, 178 cases were confirmed or suspected (25), and case reports in Connecticut increased substantially in 1998 (228 provisional, 104 confirmed, Connecticut Dept. of Public Health). These adjacent states share many of the ecologic factors that support natural maintenance of both Borrelia burgdorferi and granulocytic ehrlichiae, such as populations of the tick vector I. scapularis and reservoir rodents, including white-footed mice (P. leucopus) (19,22). The incidence of Lyme disease in Connecticut and Rhode Island has been the highest in the nation for several years, with Connecticut having a reported incidence only approximately 1.3-1.5 times higher than Rhode Island’s (26). In contrast, through 1997 there was a 24-fold difference in the incidence of reported HGE cases in the two states (Connecticut 15.9; Rhode Island 0.67). Therefore, the E. phagocytophila variants may have a competitive advantage over the EP-ha, possibly in infecting certain reservoir or vector populations. A lower incidence of EP-ha and less human disease would therefore be expected in areas where the variants predominate, since a lower proportion of ticks would harbor EP-ha.
Rickettsia rickettsii, the etiologic agent of Rocky Mountain spotted fever, was first identified in the early 1900s on the basis of its association with human disease (27). Subsequent studies of veterinary infections and tick populations identified numerous Rickettsia species closely related to R. rickettsii, all clearly members of the spotted fever group but not associated with human disease. These species include R. montana, R. rhiphicephali, R. parkeri, R. bellii, and the “east side agent” R. peacockii (28,29). Nonpathogenic rickettsiae are thought to interfere with the development of more virulent R. rickettsii in Dermacentor ticks and may be found more often in ticks than are the more virulent species (30,31). Our data suggest that a similar situation may exist among the granulocytic ehrlichiae, with both pathogenic and nonpathogenic genetic variants coexisting in nature. Isolation of the new variants will allow us to address this competitive-advantage hypothesis experimentally in both ticks and mice through the use of mixed infections in the laboratory.
Identification and use of novel gene targets more variable than the 16S rRNA gene will eventually permit better assessment of variability between strains of E. phagocytophila (32–34). Future studies should include E. phagocytophila from additional geographic areas where a substantial number of human cases of granulocytic ehrlichiosis are reported (e.g., New York, Wisconsin, Minnesota) compared with areas (e.g., New Jersey, Pennsylvania, Delaware, Maryland, California) with similar vector densities but with little or no human disease.
Acknowledgments
The authors thank Stacey Carlton, Nathan Miller, Chris Whitehouse, Susan Hiers, Katya Mason, Meaghan McKenna, Kim Bizzell, Jennifer Hatfield, Rachael Priestly, Michael Brewer, and Neeta Pardanani for technical assistance. We are grateful to the Biotechnology Core Facility of the National Center for Infectious Diseases, CDC, for the synthesis of oligonucleotides.
This study was supported in part by National Institutes of Health Grant AI 30733, the Rhode Island Public Health Partnership, and a gift from the Island Fund of the New York Community Trusts. It is Contribution Number 3787 of the Rhode Island Agricultural Experiment Station.
References
- Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, McDade JE. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–6.PubMedGoogle Scholar
- Anderson BE, Dawson JE, Jones DC, Wilson KH. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–42.PubMedGoogle Scholar
- McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–42.PubMedGoogle Scholar
- Chen SM, Dumler JS, Bakken JS, Walker DH. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–95.PubMedGoogle Scholar
- Bakken JS, Dumler JS, Chen SM, Eckman MR, Van Etta LL, Walker DH. Human granulocytic ehrlichiosis in upper midwest United States. A new species emerging? JAMA. 1994;272:212–8. DOIPubMedGoogle Scholar
- Gerwirtz AS, Cornbleet PJ, Vugia DJ, Traver C, Niederhuber J, Kolbert CP, Human granulocytic ehrlichiosis: report of a case in northern California. Clin Infect Dis. 1996;23:653–4.PubMedGoogle Scholar
- Hardalo CJ, Quagliarello V, Dumler JS. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–4.PubMedGoogle Scholar
- Petrovec M, Furlan SL, Zupanc TA, Strle F, Broqui P, Roux V, Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–9.PubMedGoogle Scholar
- Brouqui P, Dumler JS, Lienhard R, Brossard M, Raoult D. Human granulocytic ehrlichiosis in Europe. Lancet. 1995;346:782–3. DOIPubMedGoogle Scholar
- Dumler JS, Bakken JS. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–10.PubMedGoogle Scholar
- Sumption KJ, Wright DJM, Cutler SJ, Dale BAS. Human ehrlichiosis in the UK. Lancet. 1995;346:1487–8. DOIPubMedGoogle Scholar
- Foley JE, Crawford-Miksza L, Dumler JS, Glaser C, Chae J-S, Yeh E, Human granulocytic ehrlichiosis in Northern California: two case descriptions with genetic analysis of the ehrlichiae. Clin Infect Dis. 1999;29:388–92. DOIPubMedGoogle Scholar
- Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, A nested PCR assay for the detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36:1090–5.PubMedGoogle Scholar
- Belongia EA, Reed KD, Mitchell PD, Kolbert CP, Persing DH, Gill JS, Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J Clin Microbiol. 1997;35:1465–8.PubMedGoogle Scholar
- Dawson JE, Warner CK, Baker V, Ewing SA, Stallknecht DE, Davidson WR, Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odocoileus virginianus). J Parasitol. 1996;82:52–8. DOIPubMedGoogle Scholar
- Zeidner NS, Burkot TR, Massung RF, Nicholson WL, Dolan MC, Rutherford JS, Transmission of the agent of HGE by Ixodes spinipalpis ticks: Evidence of an enzootic cycle of coinfection with Borrelia burgdorferi in Northern Colorado. J Infect Dis. 2000;182:616–9. DOIPubMedGoogle Scholar
- Nicholson MC, Mather TN. Methods for evaluating Lyme disease risks using geographic information systems and geospatial analysis. J Med Entomol. 1996;33:711–20.PubMedGoogle Scholar
- Pancholi P, Kolbert CP, Mitchell PD, Reed KD, Dumler JS, Bakken JS, Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–12.PubMedGoogle Scholar
- Telford SR III, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–14. DOIPubMedGoogle Scholar
- Dear S, Staden R. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 1991;19:3907–11. DOIPubMedGoogle Scholar
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–95. DOIPubMedGoogle Scholar
- Stafford KC III, Massung RF, Magnarelli LA, Ijdo JW, Anderson JF. Infection with agents of human granulocytic ehrlichiosis, Lyme disease, and babesiosis in wild white-footed mice (Peromyscus leucopus) in Connecticut. J Clin Microbiol. 1999;37:2887–92.PubMedGoogle Scholar
- Magnarelli LA, Ijdo JW, Stafford KC III, Fikrig E. Infections of granulocytic ehrlichiae and Borrelia burgdorferi in white-tailed deer in Connecticut. J Wildl Dis. 1999;35:266–74.PubMedGoogle Scholar
- Schouls LM, Van de Pol I, Rijpkema SGT, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Statewide surveillance for ehrlichiosis—Connecticut and New York. MMWR Morb Mortal Wkly Rep. 1998;47:476–80.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Lyme disease—United States. MMWR Morb Mortal Wkly Rep. 1997;46:531–5.PubMedGoogle Scholar
- Wolbach SB. Studies on Rocky Mountain spotted fever. J Med Res. 1919;41:2–197.
- Niebylski ML, Schrumpf ME, Burgdorfer W, Fischer ER, Gage KL, Schwan TG. Rickettsia peacockii sp. Nov., a new species infecting wood ticks, Dermacentor andersoni, in western Montana. Int J Syst Bacteriol. 1997;47:446–52.PubMedGoogle Scholar
- Burgdorfer W, Hayes SF, Mavros AJ. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsiae. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and rickettsial diseases. New York: Academic Press; 1981. p. 585-94.
- Burgdorfer W. Ecological and epidemiological considerations of Rocky Mountain spotted fever and scrub typhus. In: Walker DH, editor. Biology of rickettsial diseases. Boca Raton (FL): CRC Press; 1988. p. 33-50.
- Massung RF, Owens JH, Ross D, Reed KD, Petrovec M, Bjoersdorff A, Sequence analysis of the ank gene of granulocytic ehrlichiae. J Clin Microbiol. 2000;38:2917–22.PubMedGoogle Scholar
- Storey JR, Doros-Richert LA, Gingrich-Baker C, Munroe K, Mather TN, Coughlin RT, Molecular cloning and sequencing of three granulocytic Ehrlichia genes encoding high-molecular-weight immunoreactive proteins. Infect Immun. 1998;66:1356–63.PubMedGoogle Scholar
- Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–36. DOIPubMedGoogle Scholar
Figure
Tables
Cite This Article1Since this study was conducted, new nomenclature (Anaplasma phagocytophila) has been proposed; see Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia, and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia pagocytophila. Int J Syst Evol Microbiol 2001;51:2145-65.
Table of Contents – Volume 8, Number 5—May 2002
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Thomas N. Mather, Center for Vector-Borne Disease, University of Rhode Island, 9 East Alumni Ave., Suite 7, Kingston, RI 02881, USA: fax: (401) 874-2494
Top