Volume 14, Number 6—June 2008
Letter
Coronavirus Antibodies in Bat Biologists
Figure
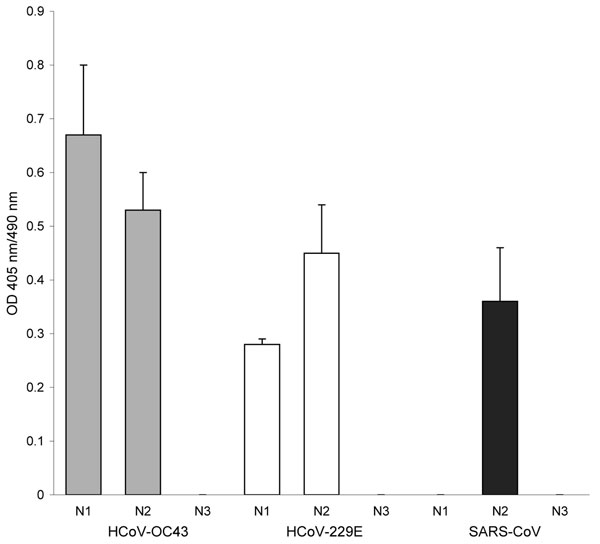
Figure. Antibody reactivity to coronavirus (CoV) nucleocapsid (N) protein fragments by ELISA. A set of recombinant protein fragments covering the N protein sequence of human CoV (HCoV)–OC43, HCoV-229E, and severe acute respiratory syndrome (SARS)–CoV were used as antigen; the serum (1:400 dilution) from the participant was tested by ELISA. The fragments include the following HCoVs: HCoV-OC43 N1 (aa 1–119), HCoV-OC43 N2 (aa 120–332), HCoV-OC43 N3 (aa 333–448), HCoV-229E N1 (aa 1–74), HCoV-229E N2 (aa 75–311), HCoV-229E N3 (aa 312–389), SARS-CoV N1 (aa 1–105), SARS-CoV N2 (aa 106–324), and SARS-CoV N3 (aa 325–422). The HCoV-OC43, HCoV-229E, and SARS-CoV fragments were coated at 4 × 10–7 M, 2.5 × 10–3 M, and 8 × 10–8 M, respectively. The N-terminal of the N protein contains a highly conserved motif (FYYLGTGP) found in all CoVs (7). This conserved motif is found in HCoV-OC43 N2, HCoV-229E N2, and SARS-CoV N2 recombinant protein fragments. The sizes of the expressed protein fragments used in this study were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. In addition, the reactivity of each protein fragment was confirmed by using Western blot with the anti-His antibody and the respective convalescent-phase serum. The mean optical density (OD) of absorbance at 405 nm (490-nm reference) of duplicate wells is shown. Error bars represent the standard deviation of duplicate wells.
References
- World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003 [cited 2005 Jul 26]. Available from http://www.who.int/csr/sars/country/table2004_04_21/en/print.html
- Li W, Shi Z, Yu M, Ren W, Smith C, Epstein JH, Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–9. DOIPubMedGoogle Scholar
- Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, Wong BH, Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A. 2005;102:14040–5. DOIPubMedGoogle Scholar
- Muller MA, Paweska JT, Leman PA, Drosten C, Grywna K, Kemp A, Coronavirus antibodies in African bat species. Emerg Infect Dis. 2007;13:1367–70.PubMedGoogle Scholar
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. DOIPubMedGoogle Scholar
- Haynes LM, Miao C, Harcourt JL, Montgomery JM, Le MQ, Dryga SA, Recombinant protein-based assays for detection of antibodies to severe acute respiratory syndrome coronavirus spike and nucleocapsid proteins. Clin Vaccine Immunol. 2007;14:331–3. DOIPubMedGoogle Scholar
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. DOIPubMedGoogle Scholar
- Sun ZF, Meng XJ. Antigenic cross-reactivity between the nucleocapsid protein of severe acute respiratory syndrome (SARS) coronavirus and polyclonal antisera of antigenic group I animal coronaviruses: implication for SARS diagnosis. J Clin Microbiol. 2004;42:2351–2. DOIPubMedGoogle Scholar
- Woo PC, Lau SK, Wong BH, Chan KH, Hui WT, Kwan GS, False-positive results in a recombinant severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid enzyme-linked immunosorbent assay due to HCoV-OC43 and HCoV-229E rectified by Western blotting with recombinant SARS-CoV spike polypeptide. J Clin Microbiol. 2004;42:5885–8. DOIPubMedGoogle Scholar
- Dominguez SR, O’Shea TJ, Oko LM, Holmes KV. Detection of group 1 coronaviruses in bats in North America. Emerg Infect Dis. 2007;13:1295–300.PubMedGoogle Scholar