Volume 14, Number 9—September 2008
Research
Pediatric Parapneumonic Empyema, Spain
Figure 2
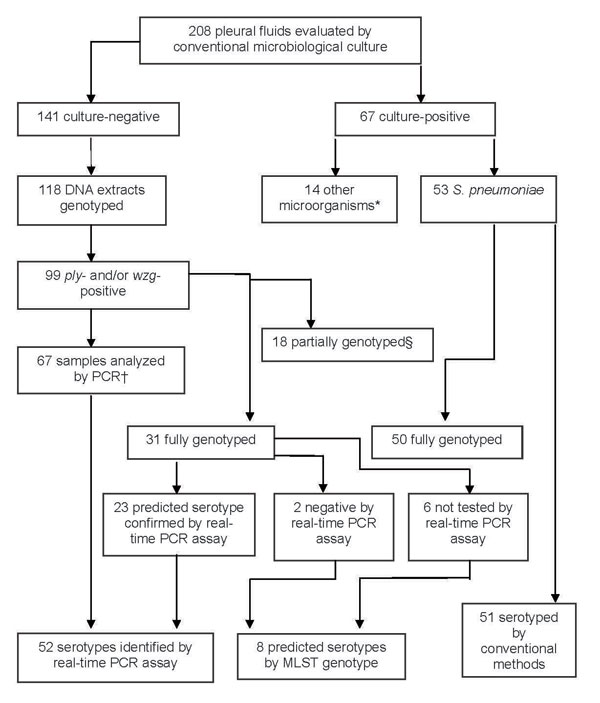
Figure 2. Microbiologic characteristics of pleural fluid (PF) specimens from pediatric parapneumonic empyema (PPE) case-patients. *Streptococcus pyogenes (6), Staphylococcus aureus (3), Mycobacterium tuberculosis (2), Escherichia coli (1), Streptococcus mitis (1), Peptostreptococcus spp. (1). †Pleural fluids analyzed by PCR included 2 samples that were ply negative but wzg positive. ‡18 partially genotyped by multilocus sequence typing (MLST) (>3 alleles), as DNA concentration was too low for reliable PCR amplification and sequencing.
Page created: July 13, 2010
Page updated: July 13, 2010
Page reviewed: July 13, 2010
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.